CARM1 small-molecule inhibitor and application thereof
A technology of small molecule inhibitors and inhibitors, applied in organic chemistry, drug combination, antineoplastic drugs, etc., can solve the problems of unsatisfactory effect, poor specificity, and low cell activity of cancer cells, and achieve low toxicity of normal cells , inhibition of proliferation, inhibition of tumor cell proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 11a
[0035] Example 1 1a, 1b preparation method:
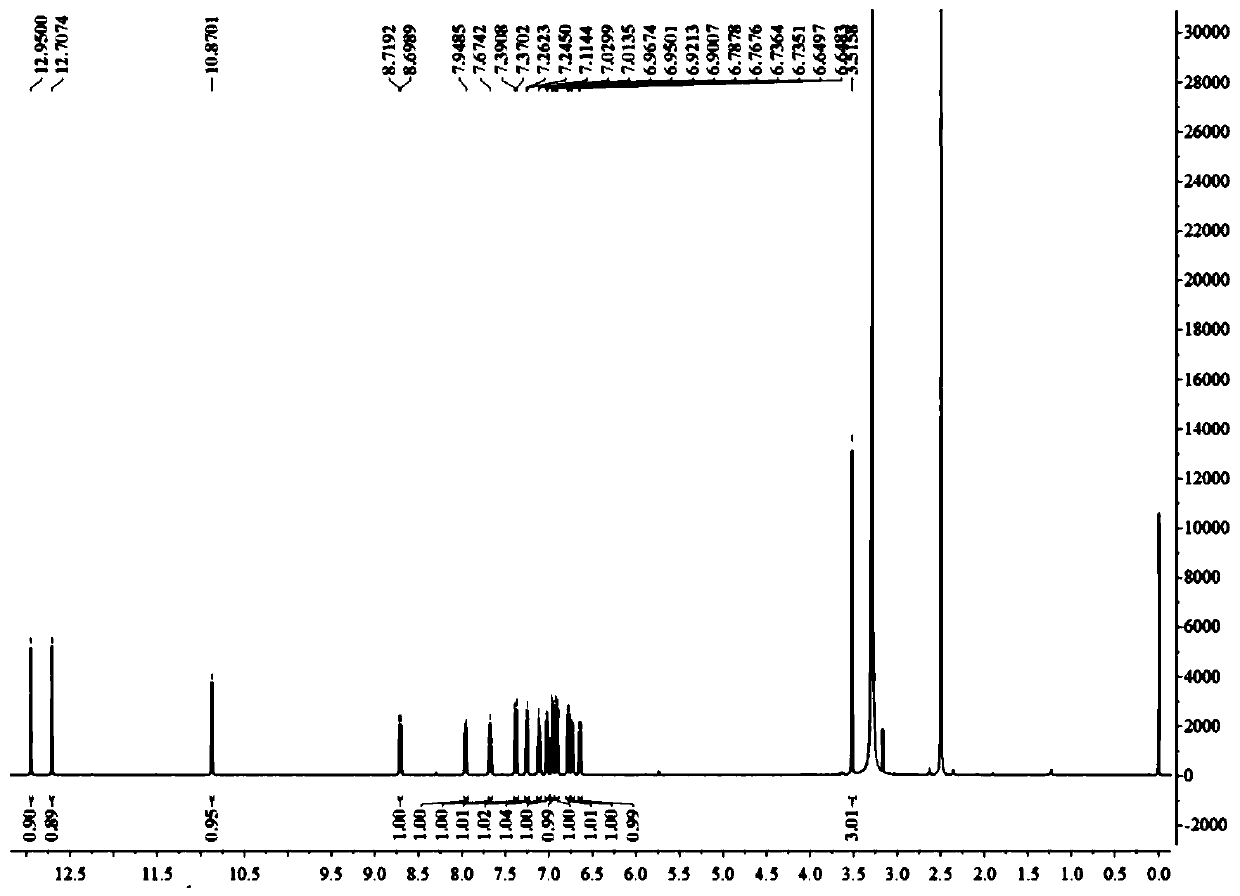
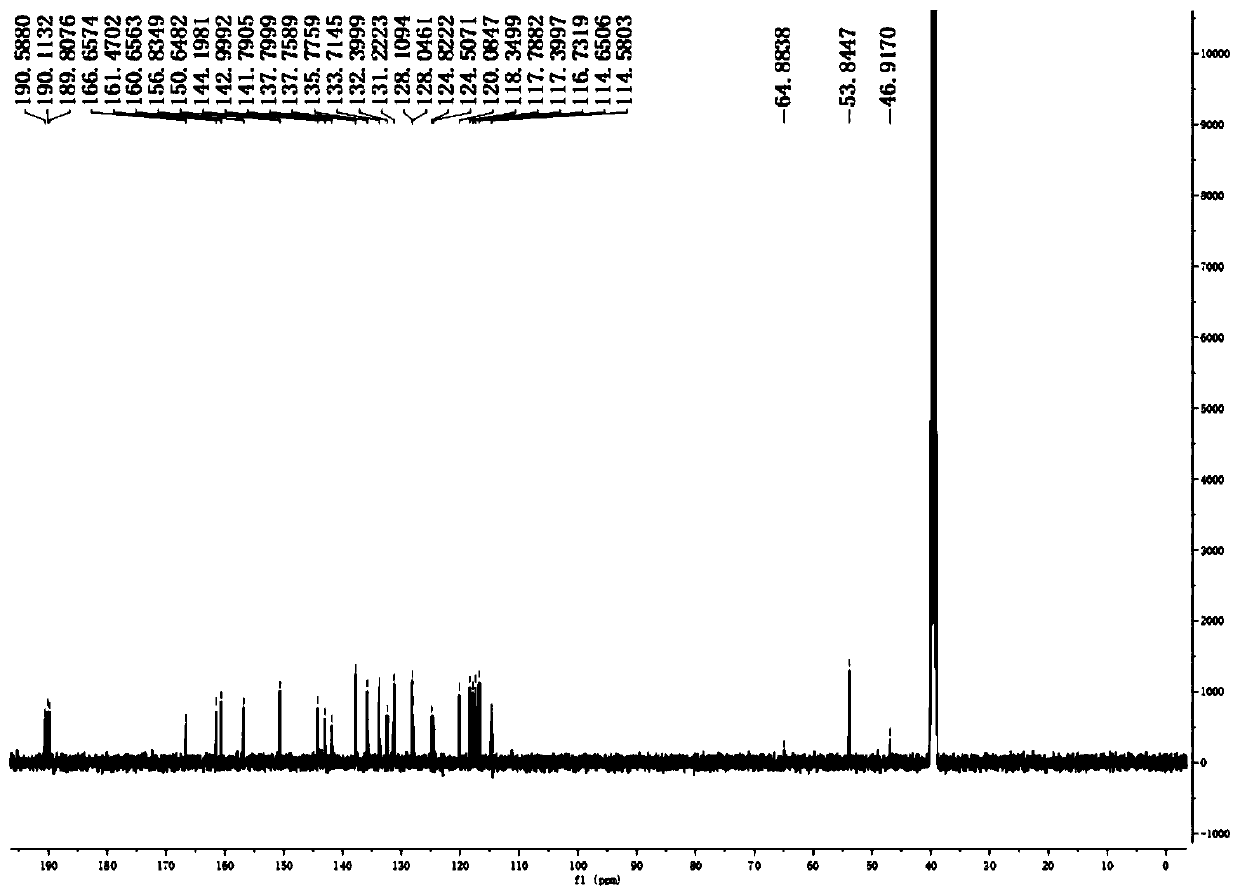
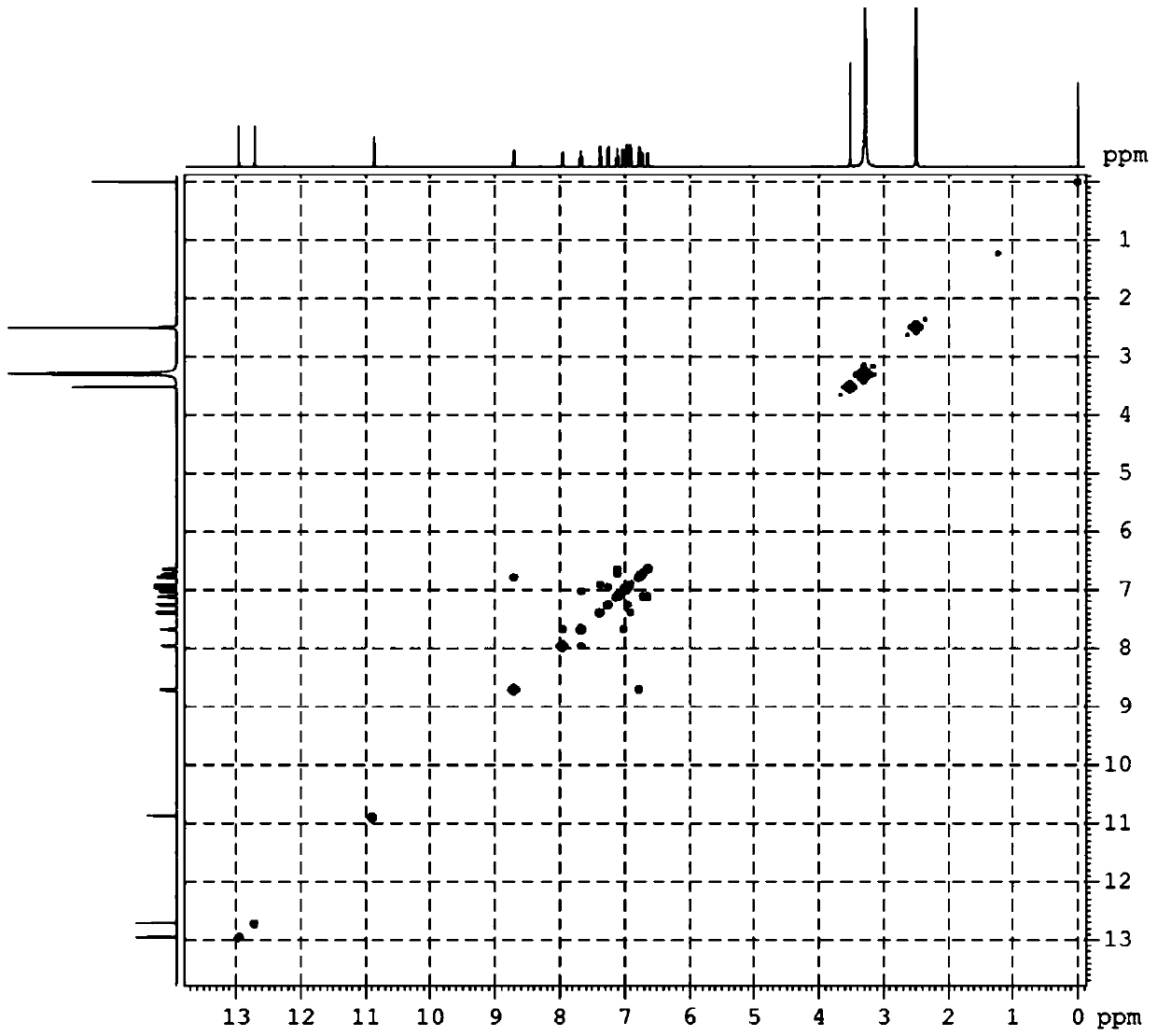
[0036] The natural products 1a and 1b are derived from the intestinal bacterium IFB-TL01 of the mantis (Tenodera aridifolia) (preserved in the China Center for Type Culture Collection, preservation number CCTCCM207198). After sonication of bacteria, a bioactive polyketide compound with a new skeleton was isolated by a bioactivity-based fractionation method (Kruzselyi D et al. Journal of Chromatographic Science, 2016, 54(7): 1084-1089.) with high resolution Electrospray ionization mass spectrometry analysis showed that the [M+Na]+ charge-to-mass ratio of the compound was 541.0897. We go further by 1 H and 13 C NMR, 1 H- 1 H COZY analysis to determine its structure ( Figure 1-3 ), single crystal X-ray crystallography analysis shows that the compound is a racemic mixture formed by a pair of enantiomers. Further, we separated the two chiral enantiomers by high performance liquid chromatography ( Figure 4 ), we named them 1a an...
Embodiment 2
[0037] Example 2 Extracellular experiments to detect the effects of 1a and 1b on the catalytic activity of CARM1
[0038] In order to verify whether 1a and 1b can inhibit the methyltransferase activity of CARM1, we first prokaryotically expressed the CARM1 protein with a GST tag and carried out methylation experiments in vitro. Figure 5 As shown, GST-CARM1 protein has methyltransferase activity, which can methylate histone H3. In addition, compared with the control, 1a and 1b can significantly inhibit the activity of CARM1 methyltransferase, and the effect of 1b is better.
[0039] Then we used microthermophoresis (MST) to detect the binding of 1b to CARM1 protein. MST uses the directional motion of particles in a microscopic temperature gradient to determine affinity by measuring changes in microthermophoresis caused by hydration layers (usually caused by changes in biomolecular structure / conformation). Image 6 As shown, in vitro binding experiments show that 1b directly ...
Embodiment 3
[0078] Example 3 Intracellular detection of the effects of 1a and 1b on the catalytic activity of CARM1
[0079] In order to verify the effect of 1a and 1b on the catalytic activity of CARM1 in cells, we used 1a and 1b to treat LNCap cells, collected the cells after 48h, extracted proteins for western blot experiments, and used different histone marker antibodies to detect related histone modifications. Variety, Figure 7 The results showed that 1a and 1b treatment of LNCap cells significantly decreased the modification level of H3R17me2a, but did not affect the modification level of H4R3me2a and H4R3me2s. Figure 8 The results indicated that 1a and 1b did not affect the protein level of CARM1. Therefore, the above experimental results confirmed that 1a and 1b can inhibit the H3R17me2a modification level in LNCap cells, and 1b showed a stronger inhibitory effect.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com