Method for detecting impurities in formoterol fumarate or related preparations thereof
A technology of formoterol fumarate and impurities, applied in measuring devices, instruments, scientific instruments, etc., can solve problems such as poor reproducibility of test results, short service life of chromatographic columns, lack of diastereoisomers, etc. Achieve excellent reproducibility, low price, and make up for the effect of diastereomer impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] Detection conditions:
[0075] Take carbooctadecylsilane bonded phase chromatographic column Waters XBridge C18 (250mm×4.6mm, 5μm); A solution is (0.6%, V / V) tetrabutylammonium hydroxide water-soluble (phosphoric acid to adjust pH=8.5), B solution It is acetonitrile, the volume ratio of solution A to solution B is 80:20; column temperature: 35°C; flow rate: 1.0mL / min; detection wavelength: 225nm; injection volume: 50μL.
[0076] Experimental steps:
[0077] Preparation of blank solution (diluent):
[0078] Measure 120ml of acetonitrile and 880ml of water respectively, shake well, and obtain.
[0079] Preparation of system suitability solution:
[0080] Weigh about 2 mg of formoterol fumarate impurity I identification reference substance (EDQM, European official reference substance), put it in a 20ml measuring bottle, add diluent to dissolve and dilute to the mark, shake well, and obtain.
[0081] Preparation of the test solution:
[0082] Take 1 bottle of formotero...
Embodiment 2
[0086] Detection condition: with embodiment 1.
[0087] Experimental steps:
[0088] The preparation of blank solution (diluent), system suitability solution, need testing solution: with embodiment 1.
[0089] Impurity I positioning solution:
[0090] Weigh about 14mg of formoterol fumarate impurity I reference substance (EDQM), put it in a 20ml measuring bottle, add diluent to dissolve and dilute to the mark, shake well, and obtain.
[0091] Impurity A positioning solution:
[0092] Weigh about 1mg of formoterol fumarate impurity A (source: TLC company) reference substance into a 100ml measuring bottle, add diluent to dissolve and dilute to the mark, shake well, and precisely pipette 1ml of the solution into a 10ml measuring bottle , dilute to the mark with diluent, shake well, and get it.
[0093] Determination:
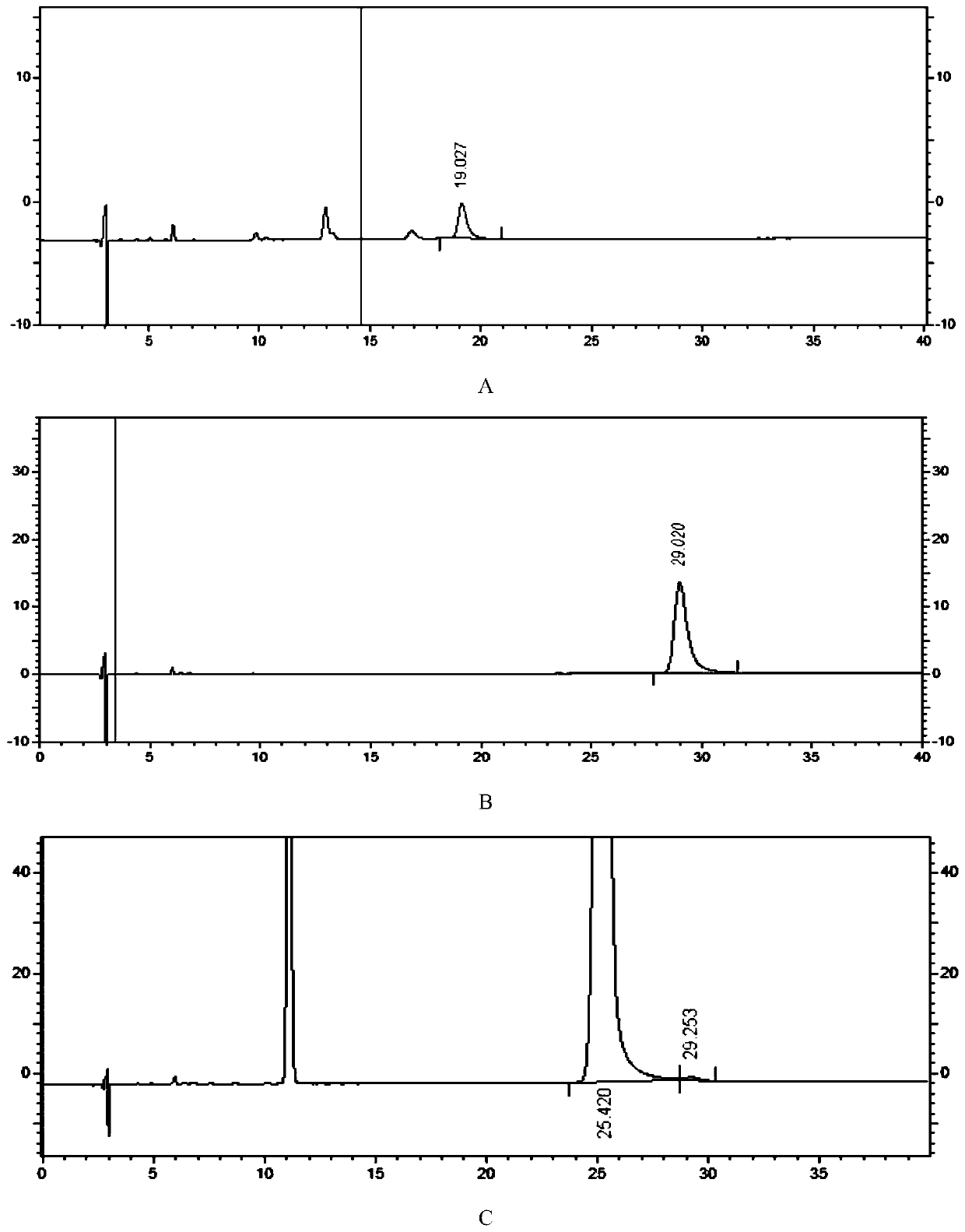
[0094] Inject one needle each of the system suitability solution, the test solution, the impurity I localization solution, and the impurity A localization sol...
Embodiment 3
[0099] Detection condition: with embodiment 1.
[0100] Experimental steps:
[0101] Preparation of blank solution (diluent) and system suitability solution: same as in Example 1.
[0102] Preparation of the test solution:
[0103] Precisely weigh 7mg of formoterol fumarate raw material, put it in a 100ml volumetric flask, add diluent to dilute and dissolve and dilute to the scale, shake well, and you get it;
[0104] Determination:
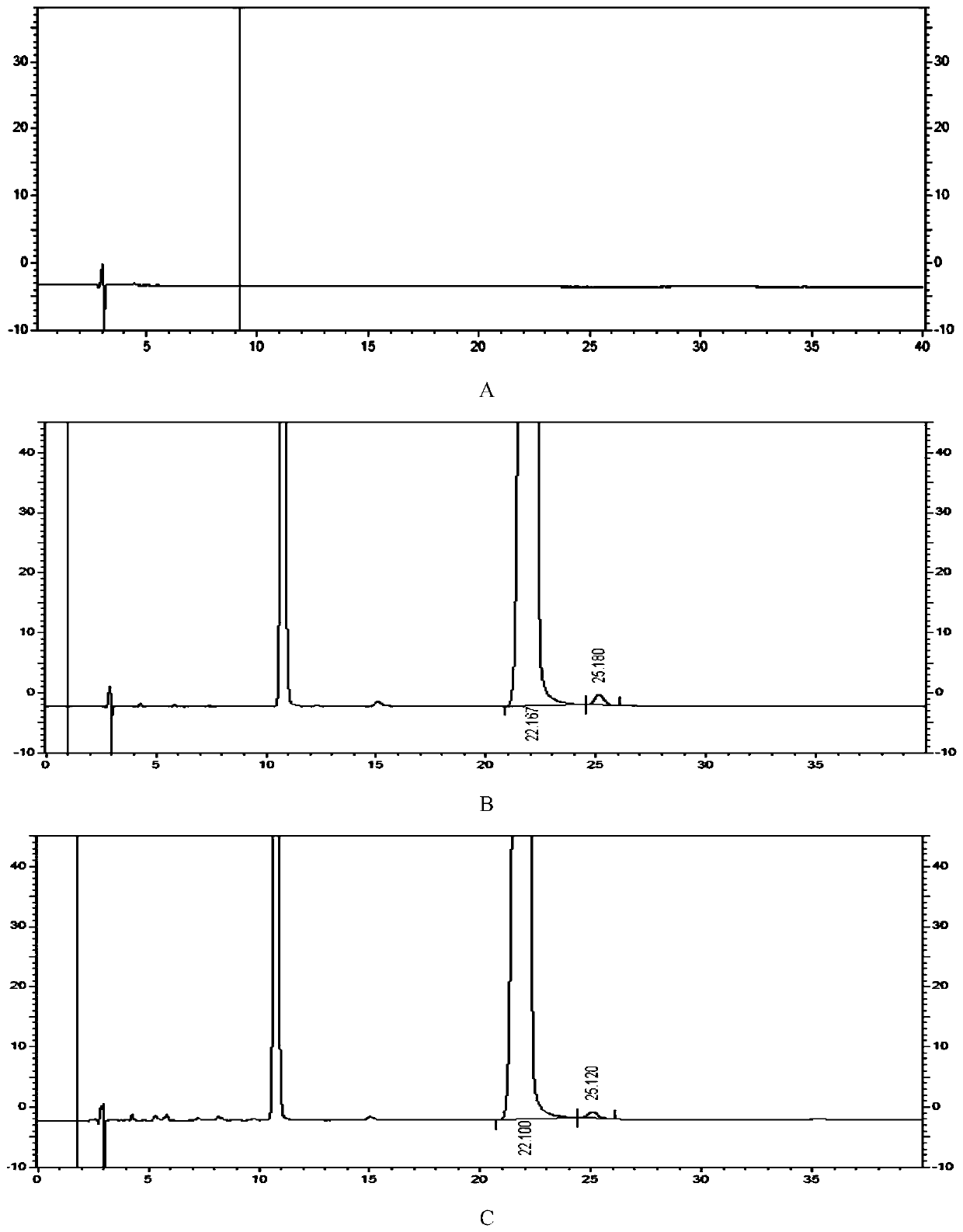
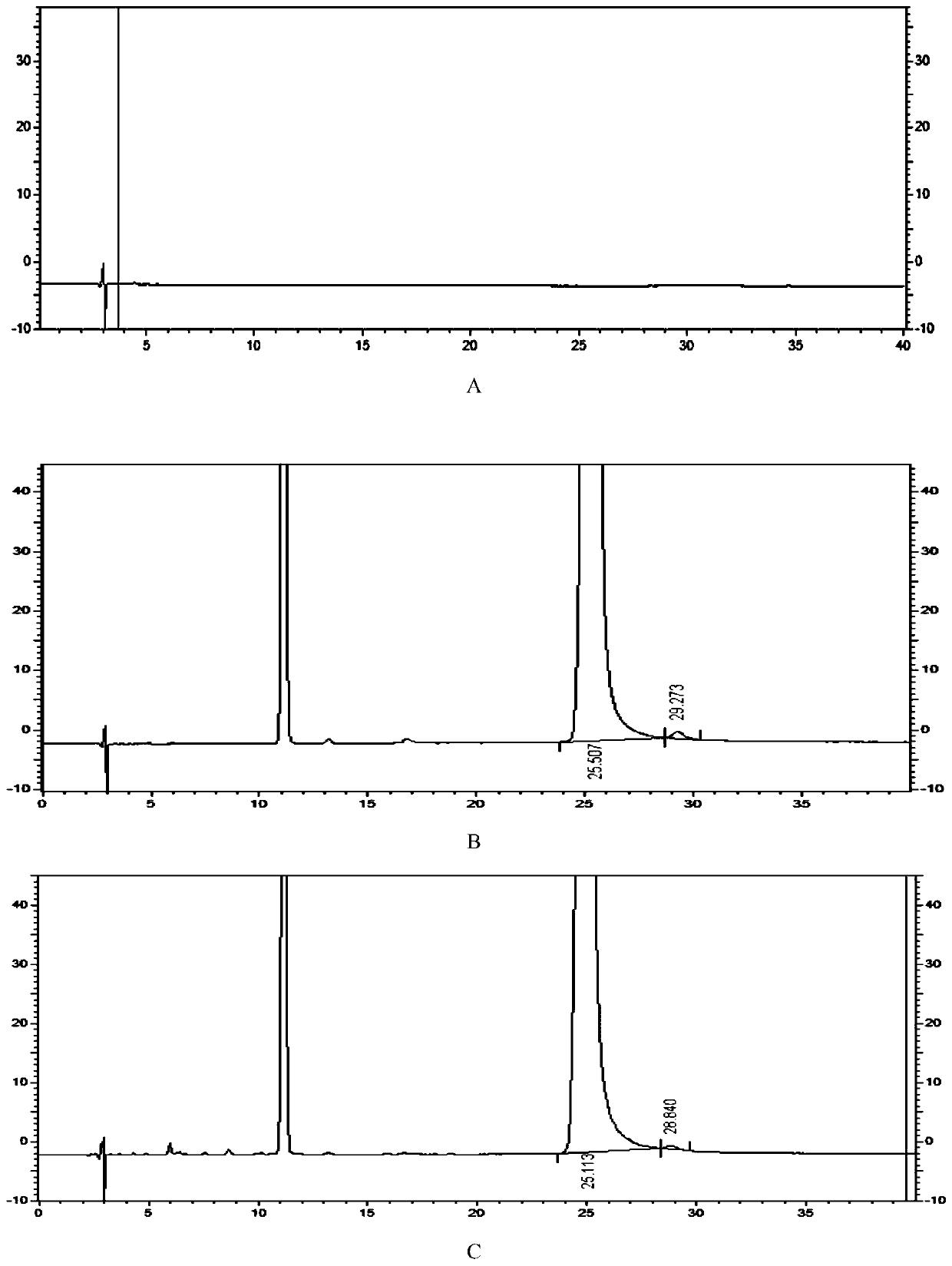
[0105] Take blank solution, system suitability solution and test solution and inject one needle each. Carry out high performance liquid chromatography analysis by above-mentioned condition, record chromatogram, calculate formoterol fumarate impurity I content by peak area normalization method. Result shows, the content of the impurity I of this batch need testing product is 0.14%, and HPLC chromatogram sees attached image 3 , image 3 A is the spectrum of the blank solution, image 3 B is the system suitability solution spectrum, image ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com