Chiral covalent organic skeleton composite film and preparation method and application thereof
A covalent organic framework and composite membrane technology, applied in chemical instruments and methods, and other chemical processes, can solve problems such as limiting chiral separation membrane capabilities, MOFs structure damage, solvent instability, etc., to achieve the applicable surface of the preparation method Wide range, simple synthesis method, uniform pore size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
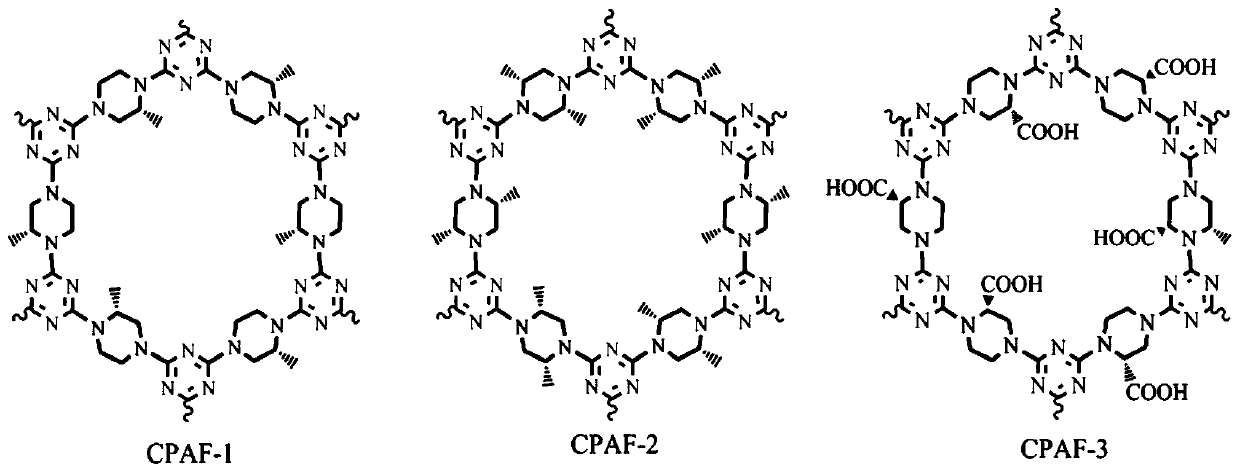
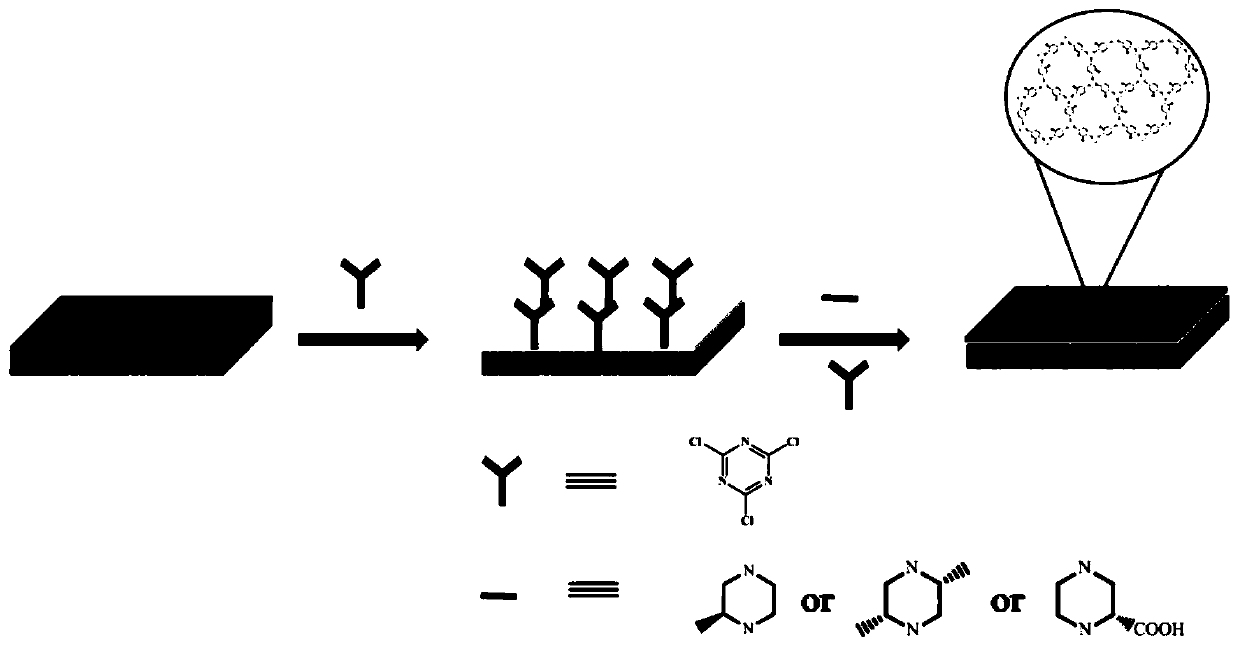
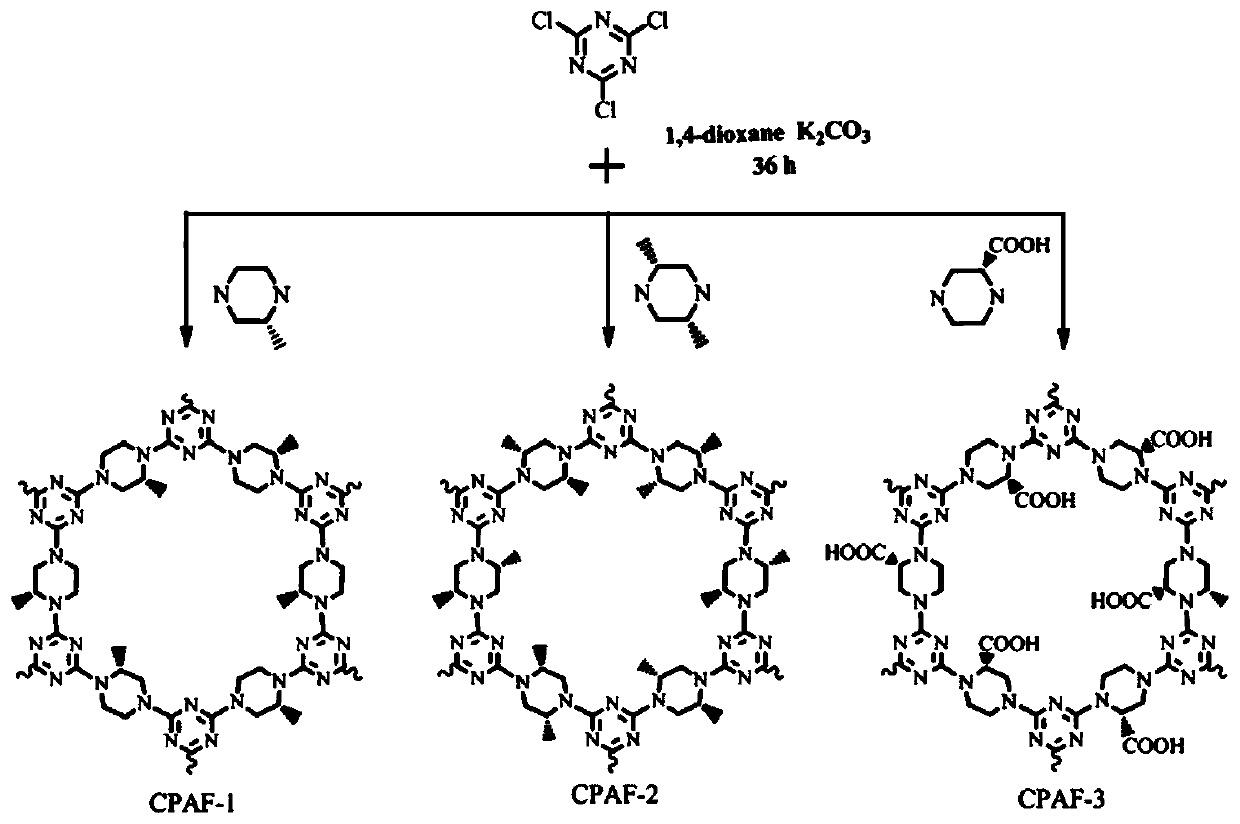
[0033] A kind of preparation method of chiral covalent organic framework composite film (CPAF-1, CPAF-2 and CPAF-3), see figure 2 and image 3 ,Specific steps are as follows:
[0034] The first step: preparation of nylon-6 membrane with surface modified cyanuric chloride:
[0035] Nylon-6 membrane is placed in a 100mL beaker, add 40mL of 0.1mol / L hydrochloric acid solution, and soak at room temperature for 24 hours. Then wash with water until neutral. Finally, soak in 40 mL of 0.1 mol / L sodium hydroxide solution for 12 hours, wash neutral with water, and dry under vacuum at 80°C for 12 hours to obtain an activated nylon-6 membrane.
[0036] The activated nylon-6 film, 0.37g of cyanuric chloride and 0.83g of potassium carbonate were reacted at 0°C for 12 hours with anhydrous 1,4-dioxane as a solvent to obtain surface-modified triuric chloride Cyanide nylon-6 membrane.
[0037] The second step: preparation of chiral covalent organic framework composite membrane:
[0038] ...
Embodiment 2
[0043] Application of the chiral covalent organic framework membrane of the present invention as a chiral separation membrane in the selective adsorption of chiral aromatic alcohol drugs
[0044] 1. The separation of RS-3-chloro-1-phenylpropanol and R / S-limonene by CPAF-1, CPAF-2 and CPAF-3 membranes as an example.
[0045] The specific chromatographic method is:
[0046] Chromatographic conditions for R / S-3-chloro-1-phenylpropanol: Chromatographic column: FLM Chiral INB (4.6×250mm, 5μm); Mobile phase: n-hexane / isopropanol (v / v=90:10); Flow rate: 0.7mL / min; column temperature: 25°C, detection wavelength: 254nm.
[0047] Chromatographic conditions of R / S-limonene: Chromatographic column: FLM Chiral INB (4.6×250mm, 5μm); mobile phase: n-hexane / isopropanol (v / v=95:5); flow rate: 1.0mL / min; column Temperature: 25°C, detection wavelength: 240nm.
[0048] 2. Separation of CPAF-1, CPAF-2 and CPAF-3 membranes, such as Figure 6 As shown, take the selective separation of R / S-3-chlo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com