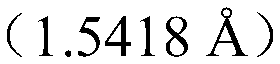Dextro-oxiracetam dispersible tablet and preparation method thereof
A technology of dispersible tablets and mass percentage, applied in the field of dextro-oxiracetam dispersible tablets and its preparation, which can solve the problems of easily decomposed impurities, large differences in tablet weight, and poor powder fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
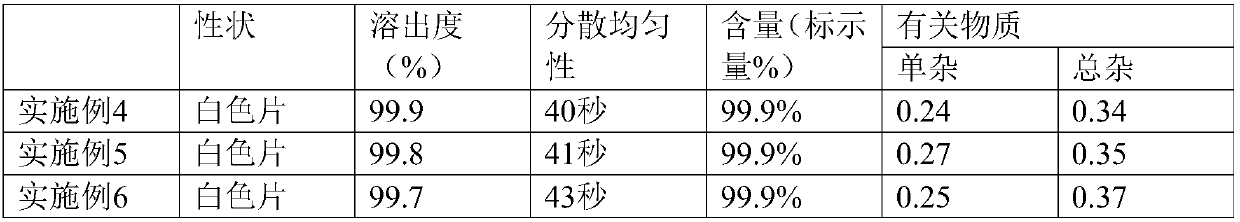
Examples
Embodiment 1
[0021] Dissolve the amorphous Dexoxiracetam at 25mg / mL with sec-butanol and heat and stir, cover and seal, stir at 35°C at a speed of 200r / min for 24h, filter, let the filtrate stand for volatilization and crystallization, and collect the crystals , dried at a temperature of 60° C. and a relative humidity of 20% for 6 hours, and collected crystals. The obtained dextro-oxiracetam crystal is subjected to powder diffraction experiment: powder diffraction determination (XRPD): test instrument condition: use Bruker D2 PHASER powder diffractometer to carry out normal temperature test, test condition is: with Cu Ka It is the light source, the voltage is 30kV, the current is 10mA, the test step is 0.014°, the scanning speed is 0.1s / step, and the scanning range is 5-40° (2θ). After testing, the prepared D-oxiracetam crystals have diffraction angles 2θ of 12.6±0.2°, 16.66±0.2°, 17.54±0.2°, 19.42±0.2°, 20.68±0.2°, 21±0.2°, 22.16± There are diffraction peaks at 0.2°, 23.46±0.2°, 25.36±0...
Embodiment 2
[0023] Dissolve the amorphous Dexoxiracetam at 10mg / mL with sec-butanol, cover and seal, stir at 40°C for 20h at a speed of 300r / min, filter, let the filtrate stand for volatilization and crystallization, collect the crystals, Dry at a temperature of 50° C. and a relative humidity of 35% for 8 hours to collect crystals. As determined by powder diffraction, the crystalline form of dexoxiracetam prepared in Example 2 is the same as the crystalline form of dexoxiracetam prepared in Example 1.
Embodiment 3
[0025] Heat and stir the amorphous dexoxiracetam at 50 mg / mL with sec-butanol to dissolve, cover and seal, stir at 25°C at a speed of 100r / min for 20h, filter, let the filtrate stand for volatilization and crystallization, and collect the crystals , dried at a temperature of 70° C. and a relative humidity of 15% for 5 hours, and collected crystals. As determined by powder diffraction, the crystalline form of dexoxiracetam prepared in Example 3 is the same as the crystalline form of dexoxiracetam prepared in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com