Preparation method of bio-based epoxy resins based on natural Magnolia officinalis derivative
A technology based on epoxy resin and epoxy resin, which is applied in the field of preparation of bio-based high-performance epoxy resin, can solve the problems of reducing the thermal stability and mechanical properties of the system, difficulty in achieving high aromatization, and poor comprehensive performance. Achieve the effect of being suitable for large-scale production, the synthesis method is simple and efficient, and easy to implement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
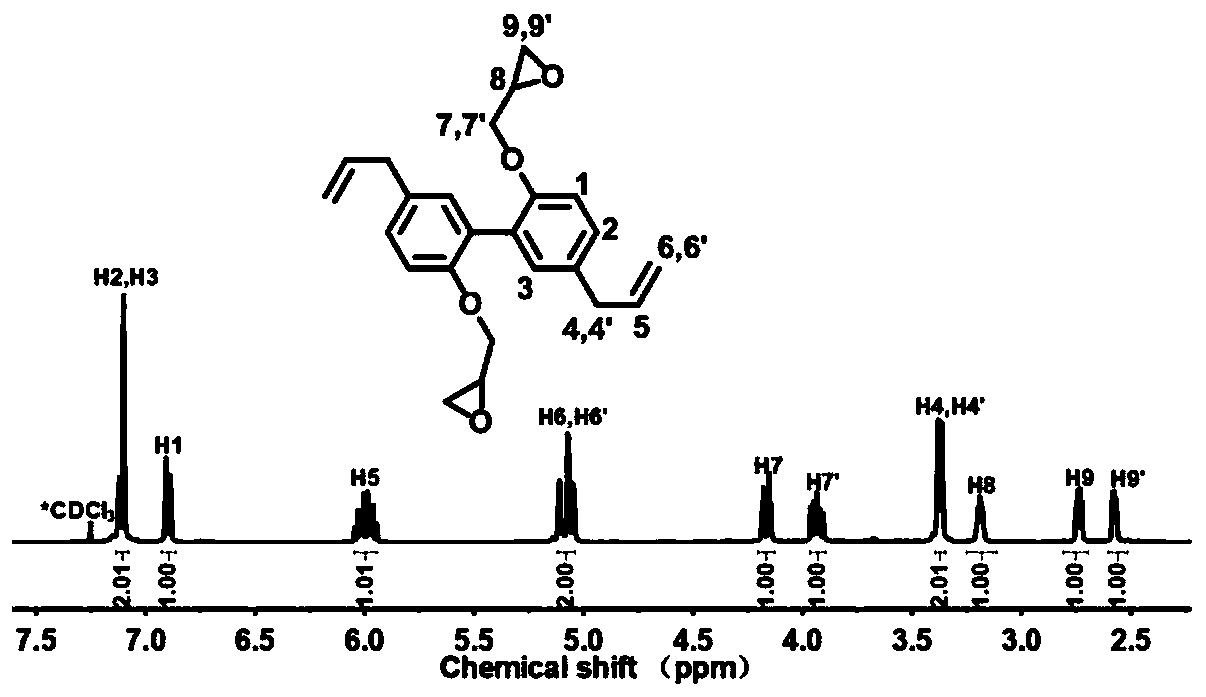
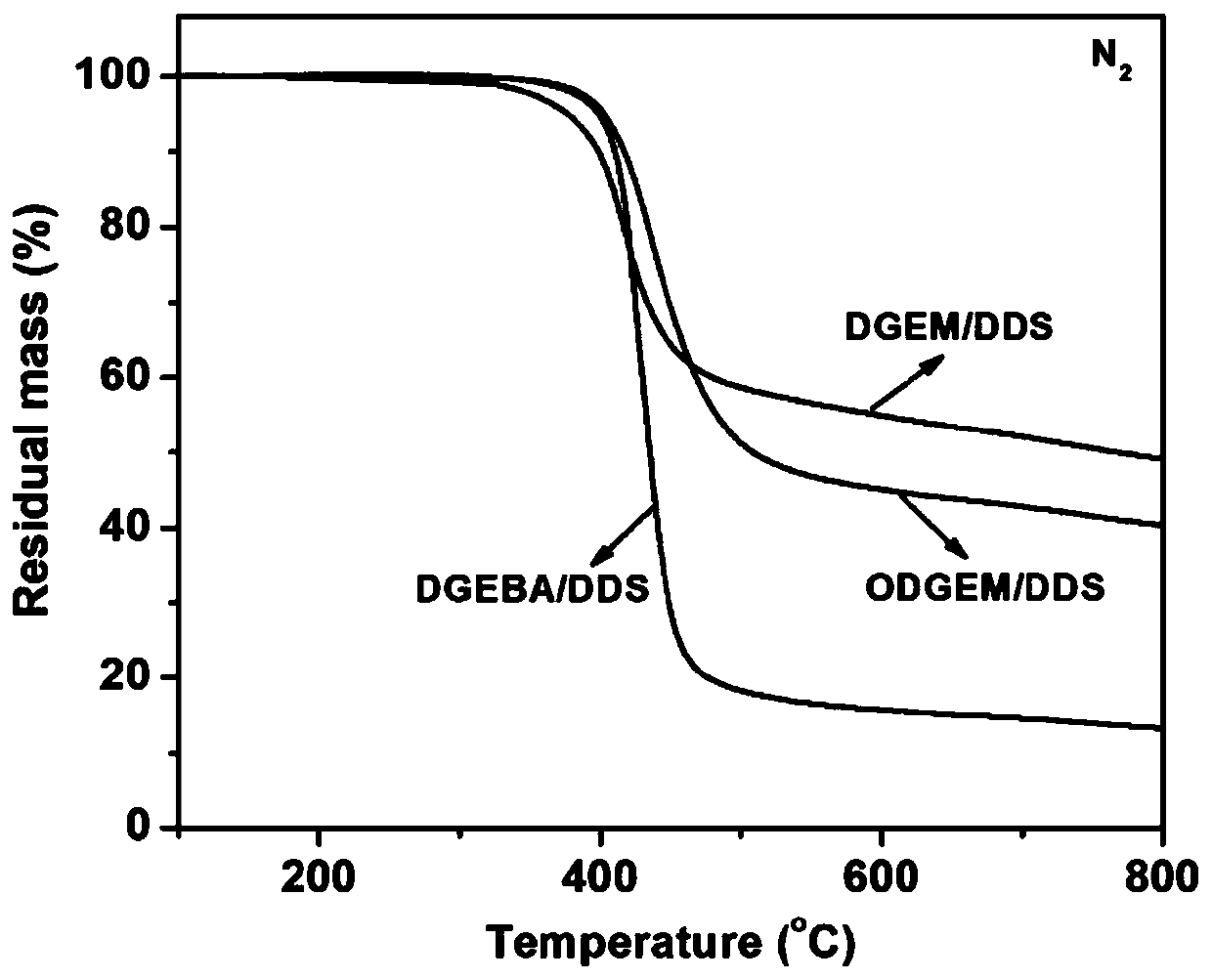
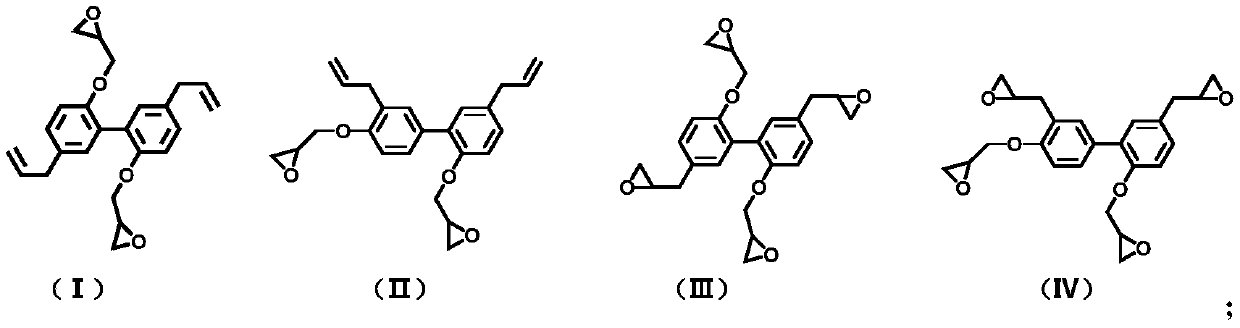
[0030](1) The synthesis of magnolol difunctionality epoxy resin: 10g magnolol, 35g epichlorohydrin, 0.42g benzyltriethylammonium chloride are added in the there-necked flask successively, under nitrogen atmosphere, be heated up to React at 100° C. for 5 hours, then take 4.5 g of sodium hydroxide to prepare an aqueous solution of sodium hydroxide with a concentration of 30 wt%, and add it dropwise into the reaction system, and continue to react for 3 hours. After the reaction, the reaction system was washed three times with distilled water, and the organic layer was separated. After drying, excess epichlorohydrin was distilled off under reduced pressure to obtain a magnolol difunctional epoxy resin.
Embodiment 2
[0032] (2) Synthesis of honokiol difunctional epoxy resin: 10g honokiol, 52g epichlorohydrin, and 2.1g benzyltriethylammonium chloride were added to a three-necked flask successively, under a nitrogen atmosphere , the temperature was raised to 120° C. for 5 hours, and then 4.5 g of sodium hydroxide was taken to form a 50 wt % sodium hydroxide aqueous solution, which was added dropwise to the reaction system, and the reaction was continued for 2.5 hours. After the reaction, the system was washed with distilled water for 3 times, and several layers were separated and dried, then distilled under reduced pressure to remove excess epichlorohydrin to obtain honokiol difunctional epoxy resin.
Embodiment 3
[0034] (3) Synthesis of magnolol tetrafunctional epoxy resin: 15g magnolol difunctional epoxy resin is dissolved in 35g methylene chloride, the system is placed in an ice-water bath, and 6.8g m-chlorine is slowly added thereto Peroxybenzoic acid, then, the system was heated to 50 ° C for 48 hours, after the reaction, the reaction system was washed with sodium sulfite aqueous solution and sodium carbonate aqueous solution for 3 times, the organic layer was separated, dried, and the solvent was removed by distillation under reduced pressure. Obtain magnolol tetrafunctional epoxy resin.
PUM
| Property | Measurement | Unit |
|---|---|---|
| flexural modulus | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com