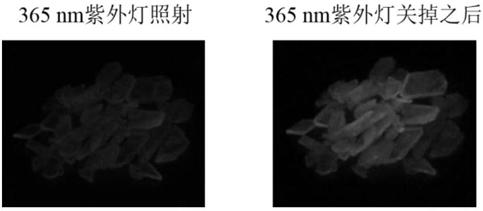
Pure organic room-temperature phosphorescent material of distorted donor-receptor structure and preparation method and application of pure organic room-temperature phosphorescent material
A room temperature phosphorescence and organic technology, which is applied in the fields of luminescent materials, organic chemistry, chemical instruments and methods, etc., can solve the problems of single molecular structure, poor luminescence performance, and lack of pure organic room temperature phosphorescence materials, etc., and achieves a simple and good preparation method. The effect of luminous performance and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] The present invention also provides a method for preparing the above-mentioned pure organic room temperature phosphorescent material, comprising:
[0051]
[0052] The compound represented by formula III is mixed with reaction raw materials, alkaline reagent and dehydrating agent, and esterified in a polar organic solvent to obtain a pure organic room-temperature phosphorescent material with a distorted donor-acceptor structure. The reaction temperature of the esterification reaction is room temperature; the reaction time of the esterification reaction is 6-24 hours.
[0053] When preparing the pure organic room temperature phosphorescent material shown in formula I, the reaction raw material is ROH; when preparing the pure organic room temperature phosphorescent material shown in formula II, the reaction raw material is OH-R'-OH; wherein, X H, F, Cl, Br or I; R is H, 1-10 carbon alkyl chain, 1-10 carbon glycol chain, phenyl, bromophenyl, iodophenyl, allyl 2-hydroxy...
Embodiment 1
[0056] Embodiment 1: the synthesis of compound A1
[0057]
[0058] Weigh 2-(9H-carbazol-9-yl)benzoic acid (402.0mg, 1.4mmol), DMAP (324.1 mg, 2.8mmol), EDCl (0.55g, 2.8mmol) and n-butanol (103.8mg, 1.4 mmol), dissolved in dichloromethane (10 mL), and stirred at room temperature for 24 hours under nitrogen protection. After the reaction, add water to the reaction solution, extract 3 times with dichloromethane, dry with anhydrous magnesium sulfate after the organic phases are combined, concentrate under reduced pressure, separate by silica gel column chromatography (eluent is sherwood oil: dichloromethane =2:1), recrystallized in n-hexane / dichloromethane mixed solvent to obtain white crystalline compound A1 (0.3 g, yield: 62.4%). Figure 16 show 1 H NMR (400MHz, CDCl 3 )δ:8.11-8.15(m,3H), 7.75(dt,J 1 =7.6Hz,J 2 =1.2Hz,1H),7.55-7.62(m,2H),7.34-7.38(m,2H), 7.24-7.27(m,2H),7.13(s,1H),7.10(s,1H),3.60( t,J=6.4Hz,2H), 0.68-0.73(m,2H),0.59-0.64(m,2H),0.52(t,J=6.8Hz,3H).HR-MS ...
Embodiment 2
[0061] Embodiment 2: the synthesis of compound A2
[0062]
[0063] The synthetic method of compound A2 is the same as that of A1, the reaction substrates are 2-(9H-carbazol-9-yl)benzoic acid (574.6mg, 2.0mmol) and n-hexanol (204.3mg, 2.0mmol), and purified to obtain White crystalline compound A2 (0.6 g, yield: 80.8%). Figure 17 show 1 H NMR (400 MHz, CDCl 3 )δ:8.11-8.15(m,3H),7.74(dt,J 1 =8.0Hz,J 2 =1.2Hz,1H), 7.55-7.62(m,2H),7.34-7.38(m,2H),7.23-7.27(m,2H),7.13(s,1H),7.11(s, 1H),3.58( t, J=6.4Hz, 2H), 0.87-1.03(m, 2H), 0.57-0.86(m, 9H). MALDI-TOF, m / z: 371.3.
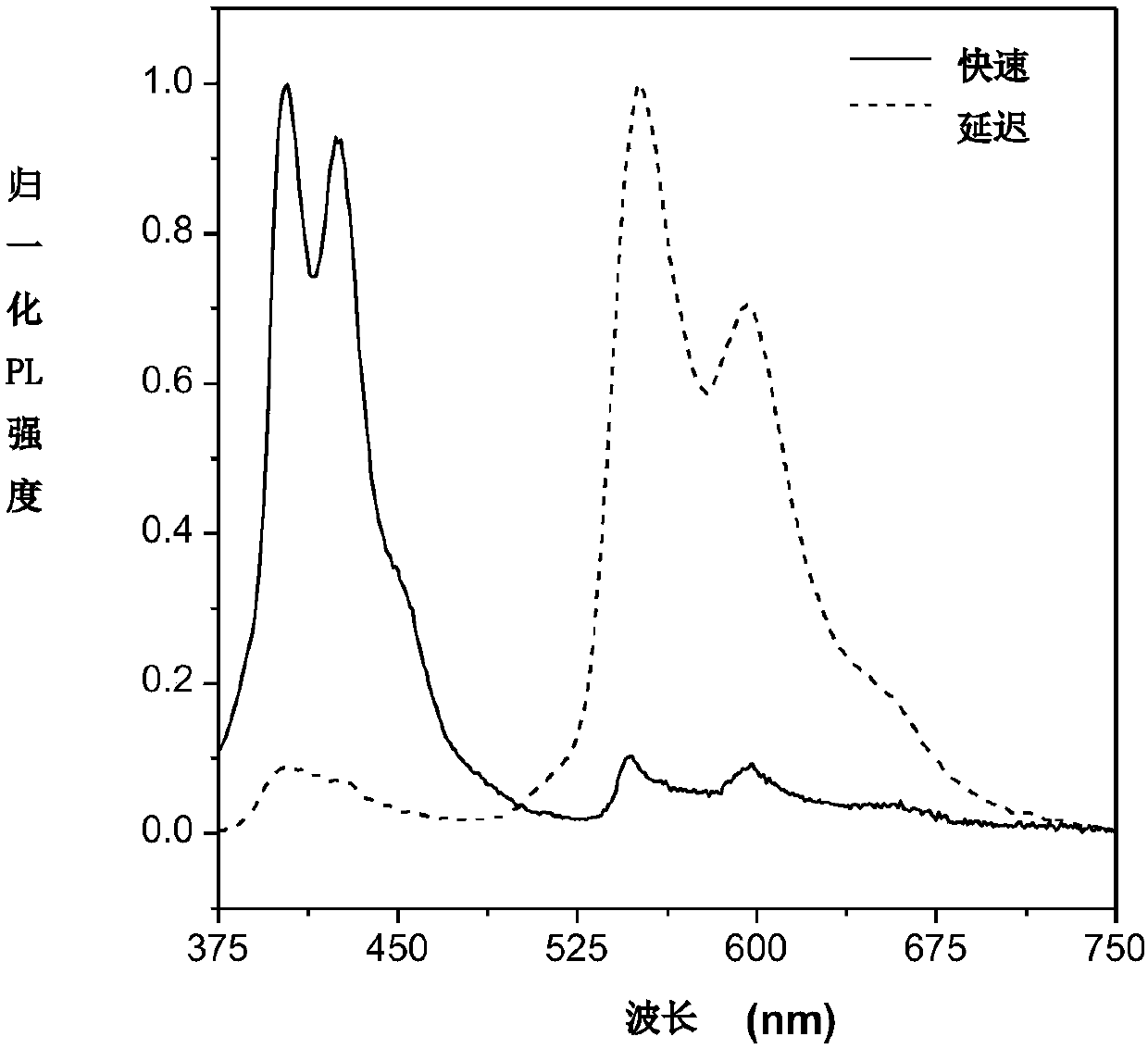
[0064] Figure 5 It is the overlay of the steady-state emission spectrum of compound A2 in the crystalline state and the steady-state emission spectrum after a delay of 10 milliseconds. The figure shows that the position of the emission peak in the steady-state emission spectrum after a delay of 10 milliseconds is consistent with the position of the emission peak at the long-wave band in the steady-state em...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Luminous life | aaaaa | aaaaa |
| Luminous life | aaaaa | aaaaa |
| Luminous life | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com