Injected amino acid derivative micromolecule gel material and application thereof
A small-molecule gel and amino acid technology, which is applied in the field of biomedical engineering in material chemistry, can solve problems such as difficult degradation and complex preparation process, and achieve the effects of convenient operation, good biocompatibility, and excellent slow-release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
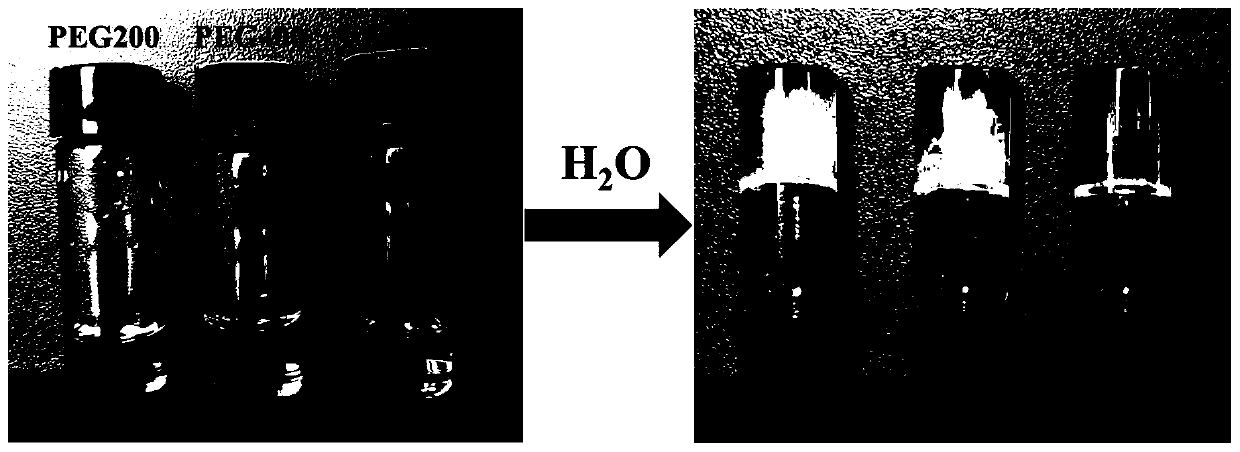
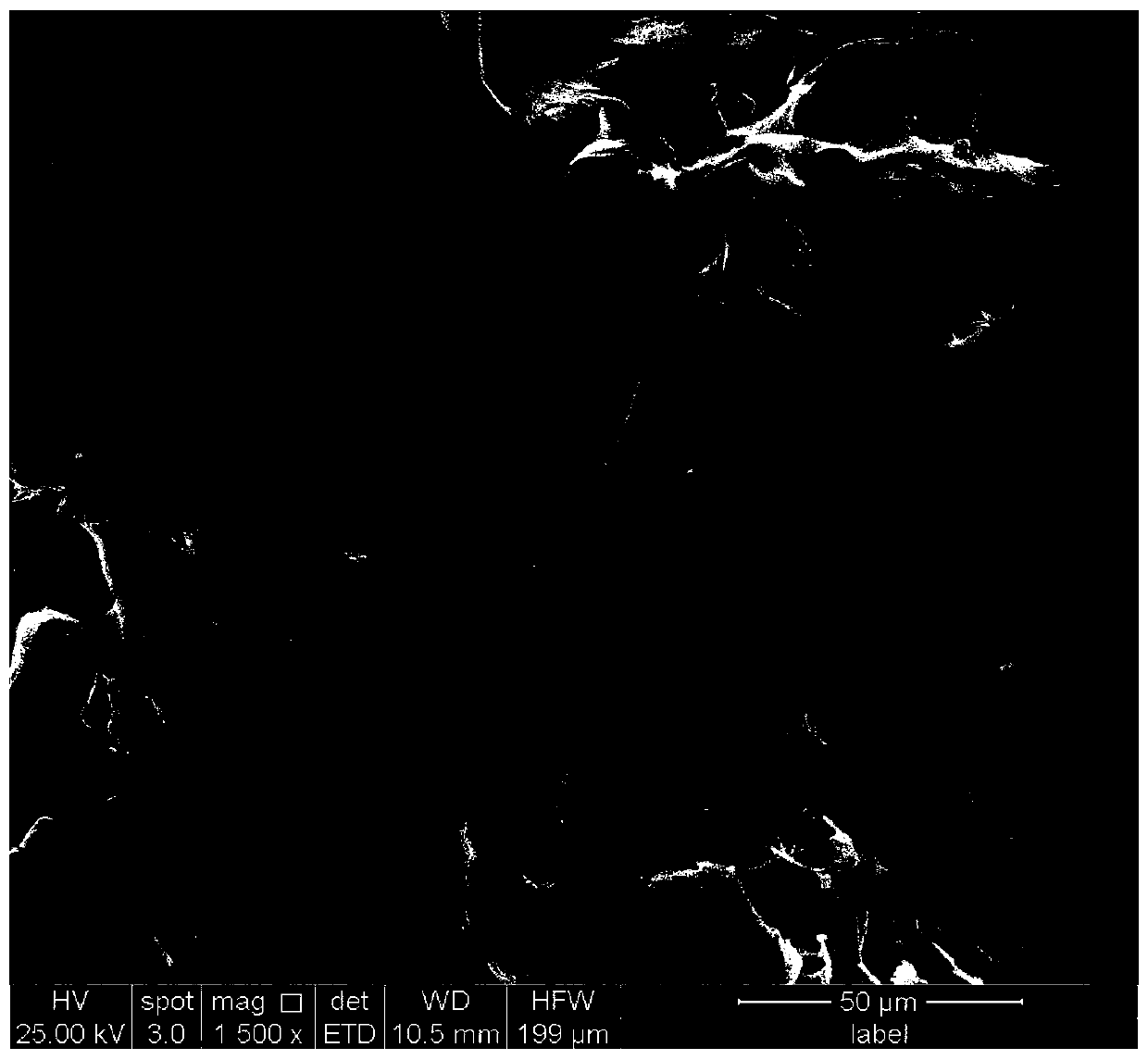
Examples
Embodiment 1
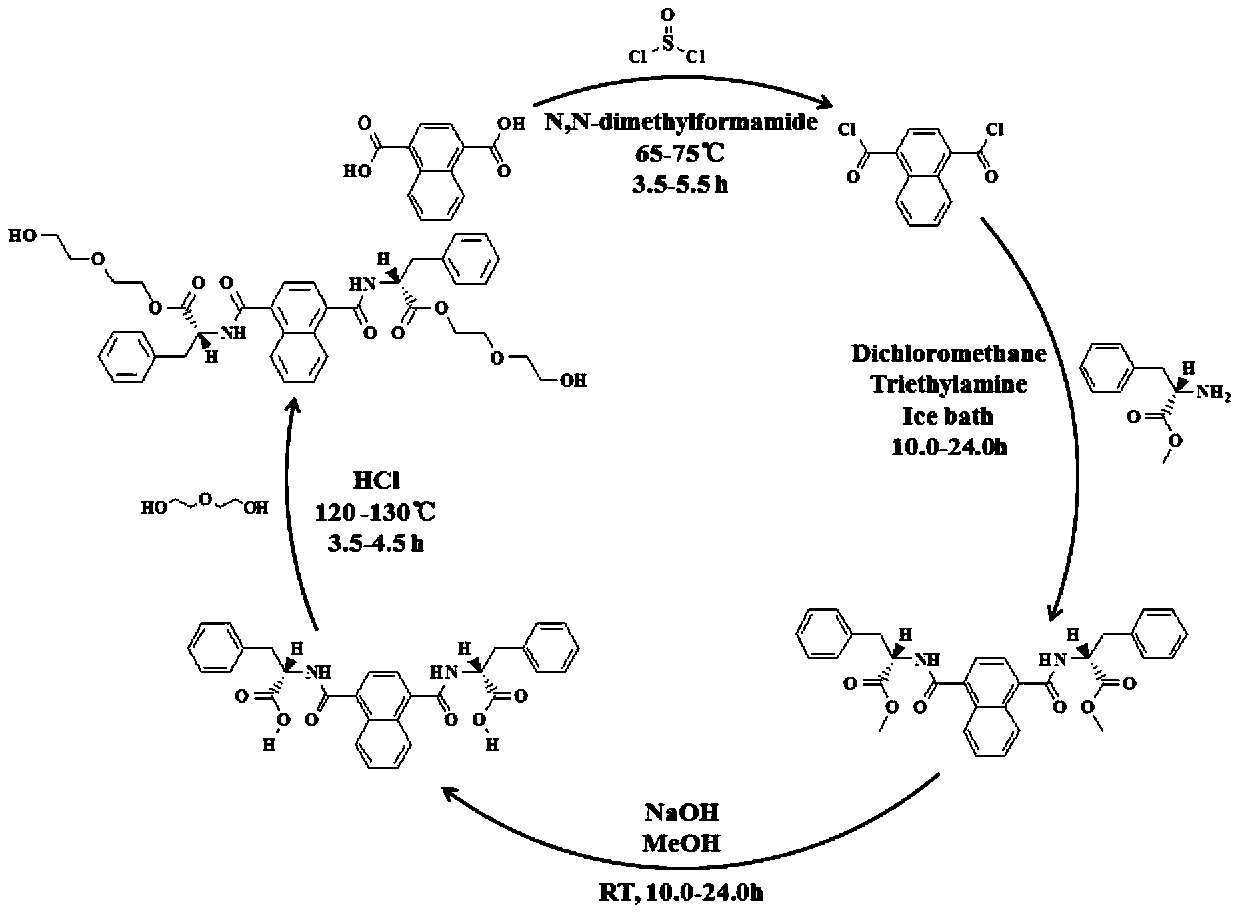
[0031] First, mix 10.00mmol 1,4-naphthalene dicarboxylic acid with 20.00mmol thionyl chloride, add 2-3 drops of DMF dropwise, and reflux at 70°C for 4.0 hours, and distill the solvent under reduced pressure to obtain 1,4-naphthalene Diformyl chloride; secondly, add 0.05mol / L 1,4-naphthalene dicarboxylic dichloride solution in dichloromethane drop by drop under ice-bath stirring to contain 0.10mol / L L-phenylalanine methyl ester and 0.13mol / L In the dichloromethane mixture of triethylamine, when the temperature rises to room temperature, continue to stir for 24.0 hours, after 35°C rotary evaporation, dichloromethane dissolution, deionized water extraction, filtration, and 35°C rotary evaporation twice, NDC is obtained. -L-PhOMe; then, prepare a methanol solution of 0.20mol / LNDC-L-PhOMe, add NaOH solution thereto (keep the concentration of NaOH in the methanol-water mixed system at 0.66mol / L), stir at room temperature for 24.0 hours, and then Acidification with HCl solution to a ...
Embodiment 2
[0038] First, mix 20.00mmol 1,4-naphthalene dicarboxylic acid with 40.00mmol thionyl chloride, add 2-3 drops of DMF dropwise, and reflux at 65°C for 5.0 hours, and distill the solvent under reduced pressure to obtain 1,4-naphthalene Diformyl chloride; secondly, add 0.08mol / L 1,4-naphthalene dichloromethane solution dropwise under stirring in an ice bath to contain 0.16mol / L D-phenylalanine methyl ester and 0.16mol / L In the dichloromethane mixture of triethylamine, when the temperature rises to room temperature, continue to stir for 15.0 hours, after 40°C rotary steaming, dichloromethane dissolution, deionized water extraction, filtration, and 40°C rotary steaming twice, NDC is obtained -D-PhOMe; then, prepare a methanol solution of 0.50mol / LNDC-D-PhOMe, add NaOH solution thereto (keep the concentration of NaOH in the methanol-water mixed system at 0.80mol / L), stir at room temperature for 15.0 hours, and then with H 3 PO 4 The solution was acidified to a pH value of 3, and th...
Embodiment 3
[0042] First, mix 30.00mmol 1,4-naphthalene dicarboxylic acid with 60.00mmol thionyl chloride, add 2-3 drops of DMF dropwise, and reflux at 75°C for 5.5 hours, and distill the solvent under reduced pressure to obtain 1,4-naphthalene Diformyl chloride; secondly, add 0.06mol / L 1,4-naphthalene dicarboxylic dichloride solution in dichloromethane drop by drop under ice-bath stirring until containing 0.14mol / L L-phenylalanine methyl ester and 0.20mol / L In the dichloromethane mixture of triethylamine, when the temperature rises to room temperature, continue to stir for 10.0 hours, after 30°C rotary evaporation, dissolving in dichloromethane, deionized water extraction, filtration, and 30°C rotary evaporation twice, to obtain NDC -L-PhOMe; then, prepare a methanol solution of 0.25mol / LNDC-L-PhOMe, add NaOH solution thereto (keep the concentration of NaOH in the methanol-water mixed system at 0.70mol / L), stir at room temperature for 10.0 hours, and then with H 2 SO 4 The solution was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com