A kind of method of synthesizing unsymmetrical cyano alkyl disulfide
A technology of cyanoalkyl disulfide and synthesis method, which is applied in the preparation of hydrogenated polysulfide/polysulfide, organic chemistry, etc., and can solve the problems of unfriendly irritating odor, difficulty in large-scale production, high price, etc. , to achieve the effect of low price, easy acquisition and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
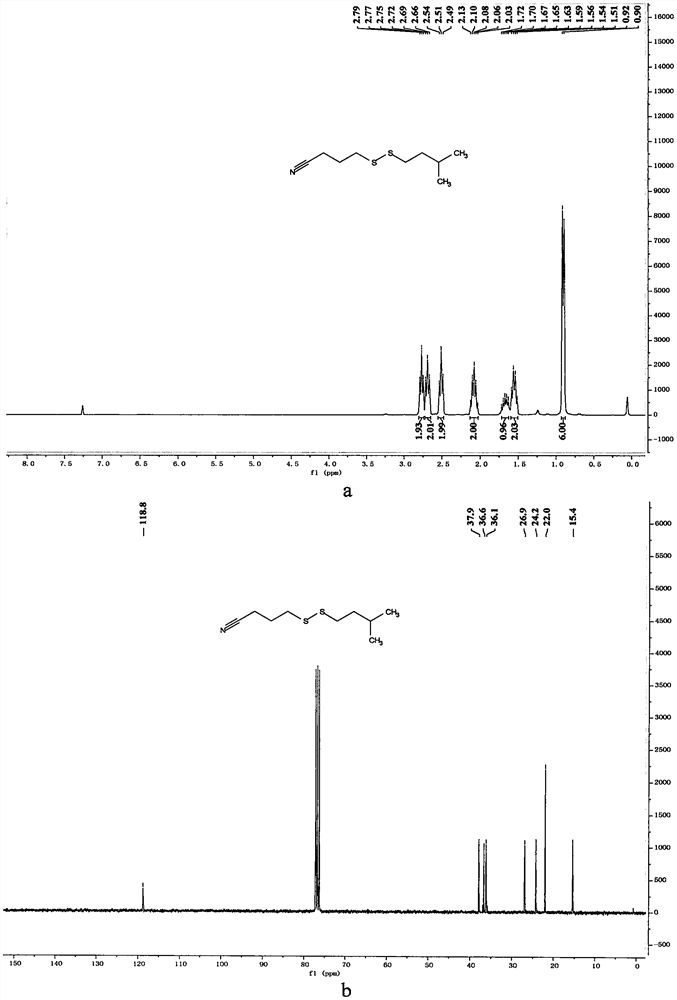
Examples
Embodiment 1
[0056] Preparation of 4-(hexyldithio)butyronitrile
[0057]
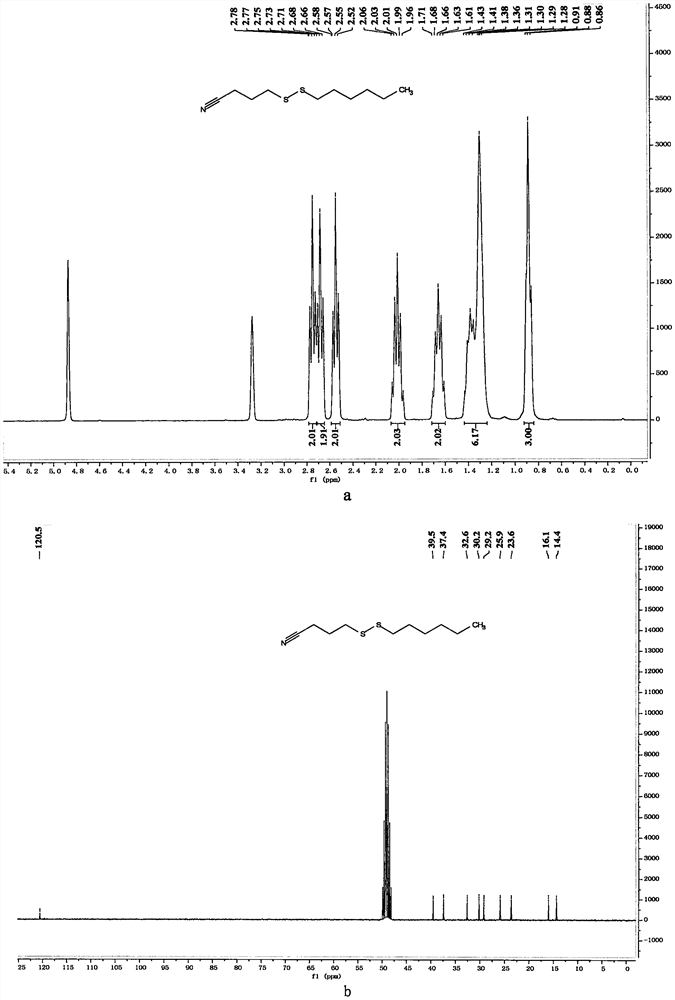
[0058] In a 10mL reaction bottle equipped with a magnet, add 3-cyanopropyl sodium thiosulfate (203mg, 1mmol, 1.25equiv.), thiourea (73mg, 0.96mmol, 1.20equiv.), sodium carbonate (102mg , 0.96mmol, 1.20equiv.), sodium dodecylbenzenesulfonate (34.8mg, 0.1mmol, 0.125equiv.), 1-bromohexane (132mg, 0.8mmol, 1equiv.) and water (1mL), in Under the protection of nitrogen, the reaction was stirred at 80° C. for 7 hours. The reaction was cooled to room temperature, 5 mL of water was added, the mixture was extracted with ethyl acetate (15 mL×3), the organic phases were combined, dried with 4 g of anhydrous magnesium sulfate, filtered through filter paper, and vacuum reduced (vacuum degree 95 mmHg, heating temperature 48 ° C) The solvent was removed, and 4-(hexyldithio)butyronitrile was obtained after separation by column chromatography (eluent polarity: petroleum ether / ethyl acetate 20:1). Yield: 83%; 1 H NMR (300MHz, CD...
Embodiment 2
[0060] Preparation of 4-(hexyldithio)butyronitrile
[0061]
[0062] In a 10mL reaction bottle equipped with a magnet, add 3-cyanopropyl sodium thiosulfate (203mg, 1mmol, 1.25equiv.), thiourea (73mg, 0.96mmol, 1.20equiv.), sodium carbonate (102mg , 0.96mmol, 1.20equiv.), sodium dodecylbenzenesulfonate (34.8mg, 0.1mmol, 0.125equiv.), 1-iodohexane (169mg, 0.8mmol, 1equiv.) and water (1mL), in Under the protection of nitrogen, the reaction was stirred at 80° C. for 7 hours. The reaction was cooled to room temperature, 5 mL of water was added, the mixture was extracted with ethyl acetate (15 mL×3), the organic phases were combined, dried with 4 g of anhydrous magnesium sulfate, filtered through filter paper, and vacuum reduced (vacuum degree 95 mmHg, heating temperature 48 ° C) The solvent was removed, and 4-(hexyldithio)butyronitrile was obtained after separation by column chromatography (eluent polarity: petroleum ether / ethyl acetate 20:1). Yield: 89%; 1 H NMR, 13 C NMR a...
Embodiment 3
[0064] Preparation of 4-(heptyldithio)butyronitrile
[0065]
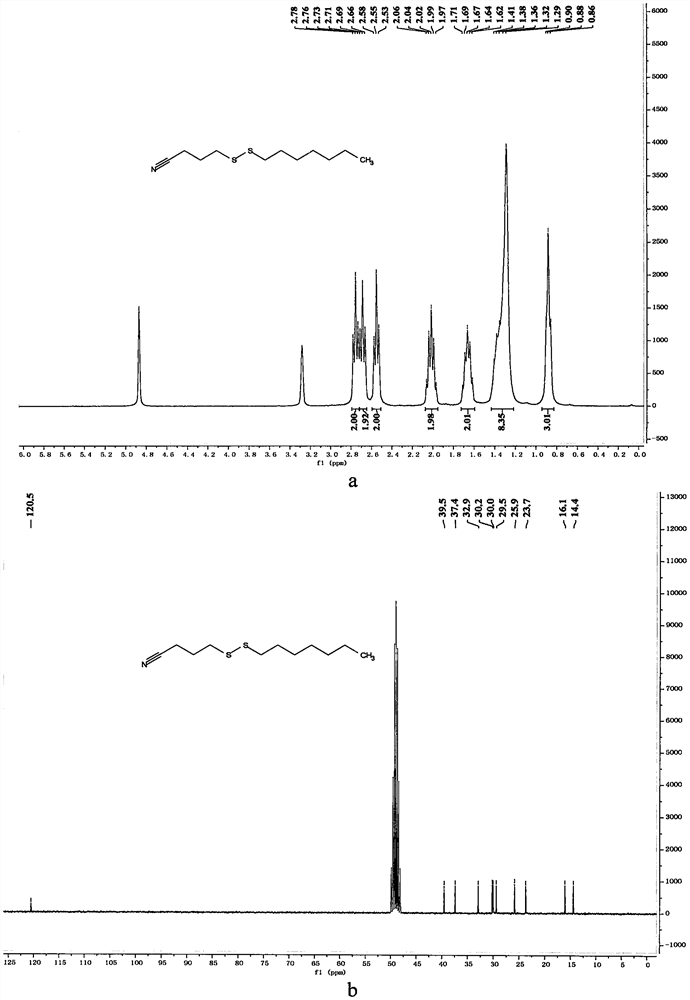
[0066] In a 10mL reaction bottle equipped with a magnet, add 3-cyanopropyl sodium thiosulfate (203mg, 1mmol, 1.25equiv.), thiourea (73mg, 0.96mmol, 1.20equiv.), sodium carbonate (102mg , 0.96mmol, 1.20equiv.), sodium dodecylbenzenesulfonate (34.8mg, 0.1mmol, 0.125equiv.), 1-bromoheptane (142mg, 0.8mmol, 1equiv.) and water (1mL), in Under the protection of nitrogen, the reaction was stirred at 80° C. for 7 hours. The reaction was cooled to room temperature, 5 mL of water was added, the mixture was extracted with ethyl acetate (15 mL×3), the organic phases were combined, dried with 4 g of anhydrous magnesium sulfate, filtered through filter paper, and vacuum reduced (vacuum degree 95 mmHg, heating temperature 48 ° C) The solvent was removed, and 4-(heptyldithio)butyronitrile was obtained after separation by column chromatography (eluent polarity: petroleum ether / ethyl acetate 20:1). Yield: 76%; 1 H NMR (300MHz,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com