Preparation method of 3-isocyanate propyltriethoxysilane
A technology of ester propyl triethoxy silane and chloropropyl triethoxy silane is applied in the field of fine chemical synthesis, can solve the problems of large operating coefficient, unsafe, low yield and the like, and achieves simple process, Simple equipment and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-6
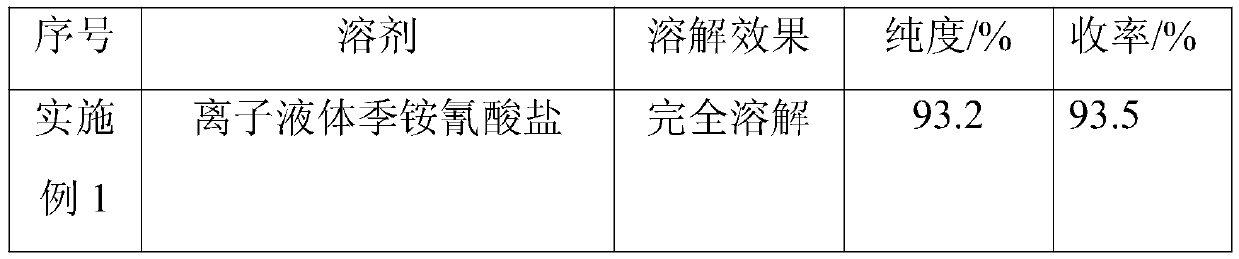
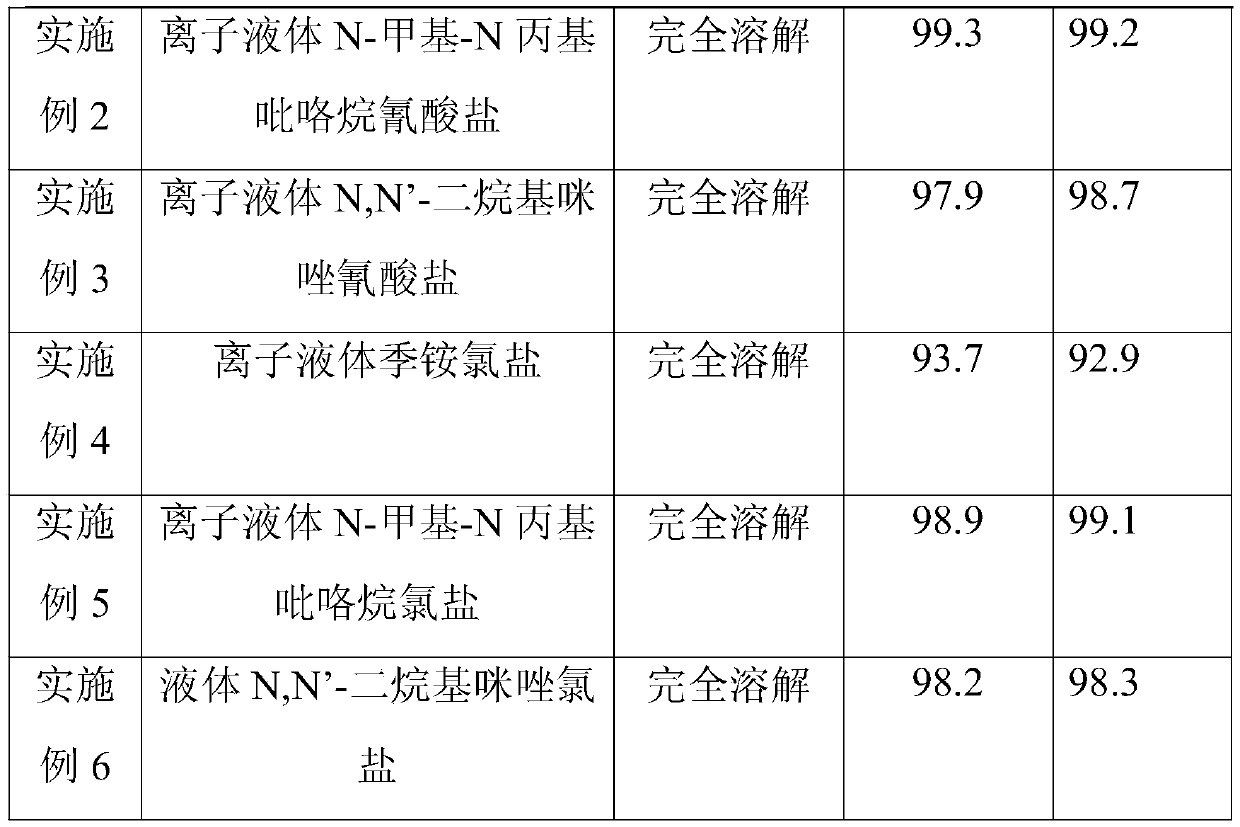
[0031] Embodiment 1-6 (the influence of different solvents on synthetic effect)
[0032] Based on the presence of a large amount of salt in the reaction system, the viscosity of the system increases; the reaction is a nucleophilic reaction, and a polar solvent needs to be added. Traditionally, polar solvents such as N,N-dimethylformamide (DMF) and N-methylpyrrolidone (NMP) are used. Sexual solvent, but its solubility for inorganic salts is weak. Therefore, a solvent with better solubility, such as ionic liquid, is used. Such as ionic liquid N-methyl-N propylpyrrolidine cyanate, ionic liquid N,N'-dialkylimidazolium cyanate, ionic liquid quaternary ammonium cyanate, ionic liquid quaternary ammonium chloride, ionic liquid N -Methyl-N-propylpyrrolidinium chloride and ionic liquid N,N'-dialkylimidazolium cyanate.
[0033] Embodiment 1-6 prepares the specific steps of 3-isocyanatopropyltriethoxysilane as follows: in the reaction flask of 3L, install mechanical stirring and tempera...
Embodiment 7-11
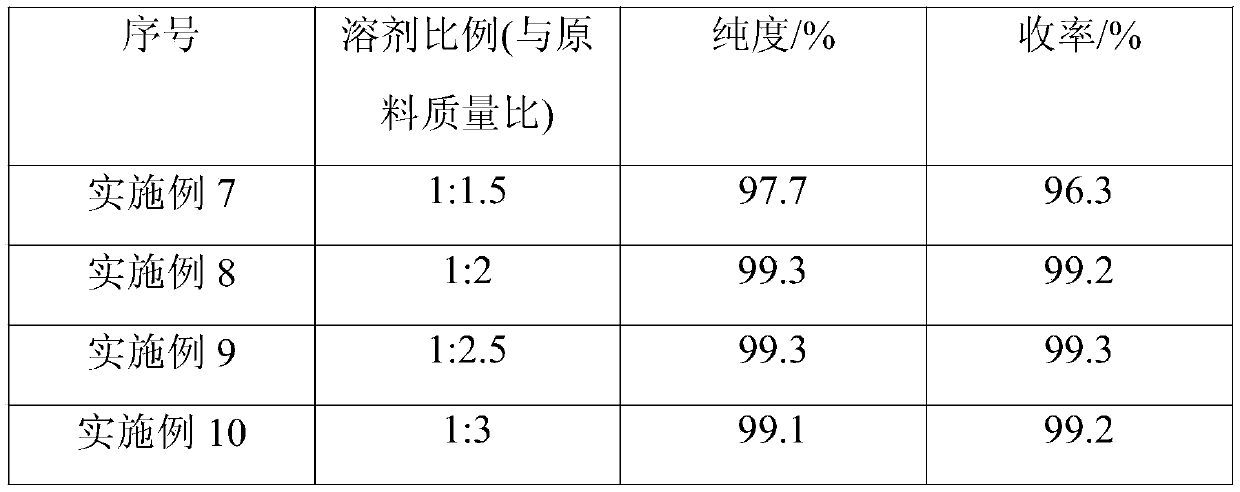
[0037] Embodiment 7-11 (the influence of different proportions of solvent on synthetic effect)
[0038] Embodiment 7-11 prepares the specific steps of 3-isocyanatopropyltriethoxysilane as follows: in the reaction flask of 3L, install mechanical stirring and temperature device, 162.2g (2mol) potassium cyanate dissolves in a certain amount of ion Liquid N-methyl-N-propylpyrrolidine cyanate, then add 9.65g catalyst, pass N 2 System replacement was carried out, and 481.6 g (2 mol) of chloropropyltriethoxysilane was added dropwise, stirring was started, and stirring was performed while adding dropwise. The reaction temperature was controlled at 100° C., and the reaction was kept for 11 hours. The reacted liquid was transferred to a rectification tower, and rectification under reduced pressure was carried out at a temperature of 170° C., and the collected fractions were analyzed by gas chromatography, and Table 2 was obtained.
[0039] Table 2
[0040]
[0041]
Embodiment 12-15
[0042] Embodiment 12-15 (the impact of different catalyst types on synthetic effect)
[0043] Embodiment 12-15 prepares the specific steps of 3-isocyanatopropyltriethoxysilane as follows: in the reaction flask of 3L, install mechanical stirring and temperature device, 162.2g (2mol) potassium cyanate and 1300g ionic liquid N- Methyl-N propylpyrrolidine cyanate, add 9.65g catalyst again, pass N 2 System replacement was carried out, and 481.6 g (2 mol) of chloropropyltriethoxysilane was added dropwise, stirring was started, and stirring was performed while adding dropwise. The reaction temperature was controlled at 100° C., and the reaction was kept for 11 hours. The reacted liquid was transferred to a rectification tower, and rectification under reduced pressure was carried out at a temperature of 170° C., and the collected fractions were analyzed by gas chromatography, and Table 3 was obtained.
[0044] table 3
[0045]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com