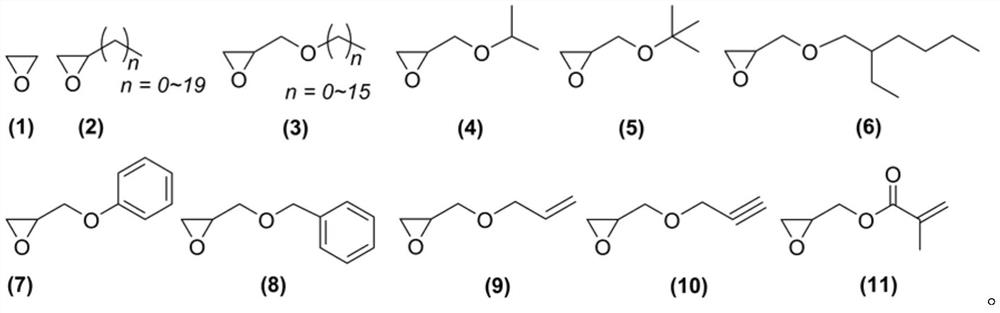
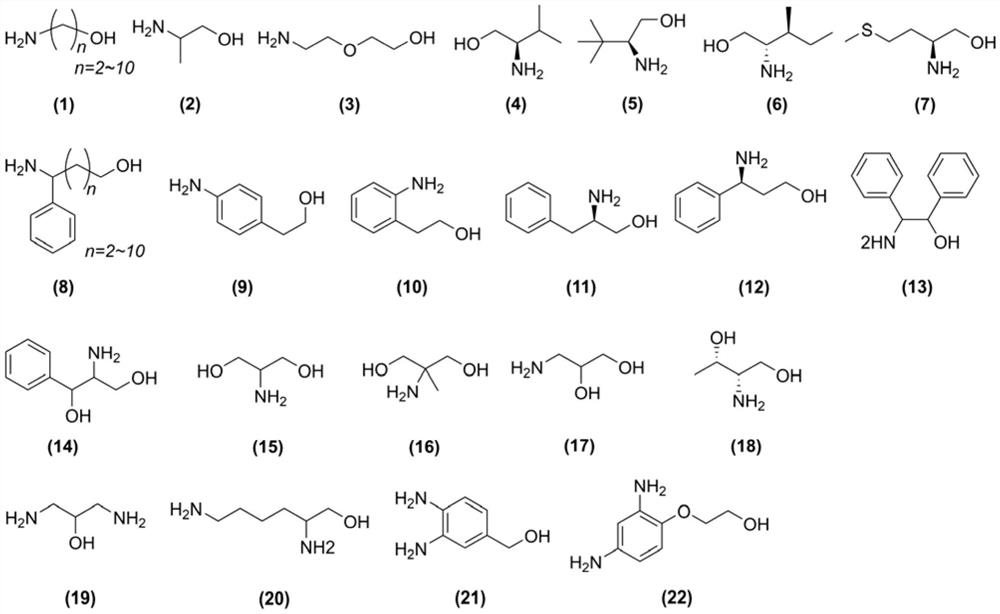
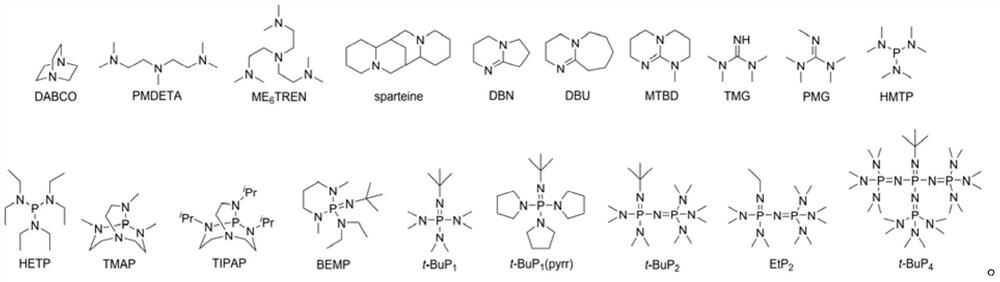
One-step synthesis method of terminal amino functionalized polyether
A technology of amino functionalization and synthesis method is applied in the field of one-step synthesis of terminal amino functionalized polyether, which can solve the problems of cumbersome deprotection process and limitations, and achieve the effects of product purity and yield improvement, high reaction efficiency and huge stock.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] In this example, alkanolamine is used as the initiator, and the non-covalent bond formed with alkyl boron is used to protect the amino group, and the hydroxyl group activated by the organic base initiates the ring-opening polymerization of ethylene oxide to prepare α-amino-ω-hydroxyl Polyethylene oxide (two-terminal heterofunctionalized PEG, NH 2 -PEG-OH). The specific operation is as follows:
[0035] In an inert atmosphere, add 1 part (mole parts) of ethanolamine into a dry glass reactor, continue to add 1.2 parts of a tetrahydrofuran solution containing triethylborane, stir and mix well. After the white mist generated during the in-situ formation of non-covalent bonds between amino groups and triethylboron dissipated, continue to add 0.005 parts of phosphazene base t-BuP 1 solution in tetrahydrofuran. In this embodiment, the molar ratio of alkylboron to amino group is 1.2, and excess alkylboron forms Lewis acid-base pair with organic base, and the molar ratio is 4...
Embodiment 2
[0038] In this embodiment, the organic base is replaced by the tertiary amine DABCO, and the others are the same as in Embodiment 1. Reaction at room temperature for 10h, that is. Theoretical number average molecular weight M of polyethylene oxide n,th It is 3.1kg / mol. The molecular weight measured by SEC was 3.4 kg / mol, and the degree of dispersion was 1.09.
Embodiment 3
[0040] In this embodiment, the organic base is replaced by triaminophosphine HMTP, and the others are the same as in Embodiment 1. Reaction at room temperature for 24h, that is. Theoretical number average molecular weight M of polyethylene oxide n,th It is 3.1kg / mol. The molecular weight measured by SEC was 3.4 kg / mol, and the degree of dispersion was 1.10.
PUM
| Property | Measurement | Unit |
|---|---|---|
| dispersity | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com