Beta-agarase, and gene and application thereof
An agarase, gene technology, applied in the application, genetic engineering, plant genetic improvement and other directions, can solve the problems of limited application, poor thermal stability and pH stability, loss of enzyme activity, etc., to achieve excellent genetic resources and enzyme resources, Wide pH range, the effect of maintaining enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The acquisition of embodiment 1β-agarase gene agaM2 gene
[0032] The β-agarase gene in this example is obtained by analyzing the marine sediment microbial metagenomic sequencing data set, named agaM2 gene, and its nucleotide sequence is the sequence shown in SEQ ID NO:2. The whole sequence of the agaM2 gene is synthesized to obtain the β-agarase gene agaM2 gene.
Embodiment 2
[0033] Cloning expression of embodiment 2 agaM2 gene
[0034] The agaM2 gene synthesized in Example 1 was linked to pEASy @ -Blunt E1 vector (purchased from full gold), the connection method was carried out according to the instructions of the vector to obtain the pEASy-agaM2 recombinant vector; the obtained recombinant vector was transformed into Escherichia coli E.coli BL21 (DE3) competent cells, and coated with Spread on LB solid culture plates containing 100 μg / mL ampicillin, culture overnight at 37°C; inoculate positive clones into 2×YT liquid medium containing 100 μg / mL ampicillin and 0.7% glucose, and culture at 37°C until bacterial liquid OD 600 When the concentration is 0.6, add isopropylthio-β-D-galactoside (IPTG) at a final concentration of 1 mM, and induce the expression of the target protein rAgaM2 after 10 h at 25°C.
Embodiment 3
[0035] The separation and purification of embodiment 3rAgaM2 protein
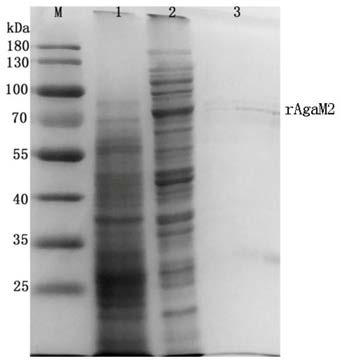
[0036] The bacterial cells obtained by inducing expression in Example 2 were collected by centrifugation, resuspended in lysis buffer (0.5mol / L NaCl, 20mmol / L Tris-HCl, pH 8.0) for lysis, and the lysed suspension was lysed at 4°C Centrifuge at 4000 rpm for 20 min, and collect the supernatant. The supernatant was previously mixed with NiSO 4 Combined R10-Flammable (purchased from GE Healthcare) was mixed and eluted with imidazole at a concentration of 200mM to obtain the recombinant β-agarase rAgaM2 protein, whose amino acid sequence is shown in SEQ ID NO.1, and carried out by SDS-PAGE Electrophoresis analysis, electrophoresis test results see figure 1 , the third lane is the purified rAgaM2 protein, and its size is consistent with the predicted molecular weight (83.5kD).
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com