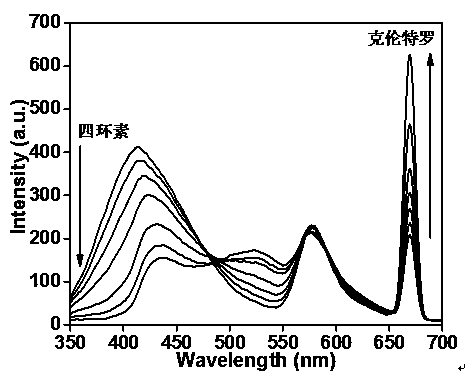
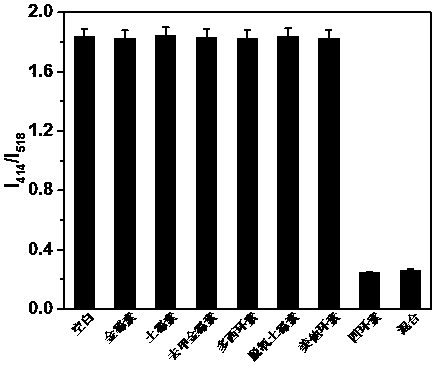
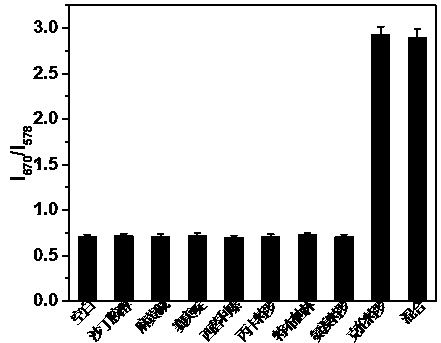
Method for detecting tetracycline and clenbuterol by carbon dot-rhodamine B bifluorescence system proportional fluorescence probe
A technology of fluorescent probe and tetracycline, which is applied in the field of chemical analysis and detection, can solve problems such as the impact on the health of the food population, antibiotic residues, and the impact on detection accuracy, and achieve high sensitivity, good specificity, and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: Determination of tetracycline and clenbuterol in pork The operation steps are as follows:
[0023] 1. Carbon dots are prepared by the following method: Weigh 1.0~5.0g of phenylboronic acid, add it to 100~150 mL of absolute ethanol, ultrasonicate for 5~10 minutes to form a colorless and transparent solution, add 4~8g of 3-aminopropyl tris Ethoxysilane, 2 ~ 4 mL ammonia water, ultrasonic 10min to form a uniform solution, and transferred to a polytetrafluoroethylene reaction kettle, heated at 180 ℃ for 8 ~ 10h, after natural cooling, centrifuged at 10000r / min for 10min, first used Filter with a pore size of 0.22 μm membrane, and then use a dialysis bag with a molecular weight cut-off of 3000-3500 Da for dialysis treatment for 24 hours to obtain fluorescent carbon dots;
[0024] 2. Preparation of carbon dots-rhodamine B fluorescent system: Take 1 mL of the above synthetic fluorescent carbon dots and mix with 0.1-0.2 mL of 0.1-0.5 mg / mL rhodamine B absolute eth...
Embodiment 2
[0036] Example 2: Determination of tetracycline and clenbuterol in chicken The operation steps are as follows:
[0037] 1. Carbon dots are prepared by the following method: Same as Example 1;
[0038] 2. Preparation of carbon dot-rhodamine B fluorescence system: same as Example 1;
[0039] 3, tetracycline standard curve making: with embodiment 1;
[0040] 4. Preparation of clenbuterol standard curve: same as in Example 1;
[0041] 5. Determination of tetracycline and clenbuterol in chicken samples:
[0042] (1) Extraction: same as Example 1;
[0043] (2) purification: same as embodiment 1;
[0044] (3) Determination of tetracycline and clenbuterol: the operation was the same as in Example 1, the content of tetracycline calculated from the regression equation was 1.23 μg / kg, and the relative standard deviation was 1.9%; clenbuterol was not detected.
Embodiment 3
[0045] Example 3: Determination of tetracycline and clenbuterol in milk samples The operation steps are as follows:
[0046] 1. Carbon dots are prepared by the following method: Same as Example 1;
[0047] 2. Preparation of carbon dot-rhodamine B fluorescence system: same as Example 1;
[0048] 3, tetracycline standard curve making: with embodiment 1;
[0049] 4. Preparation of clenbuterol standard curve: same as in Example 1;
[0050] 5. Determination of tetracycline and clenbuterol in milk samples:
[0051] (1) Milk sample preparation: take 5 mL of milk, add 10 mL of methanol-water-formic acid (28:72:0.1, v / v) extract and 1 mL of 4% trichloroacetic acid in acetonitrile, vortex for 3 min, and sonicate for 5 min, centrifuge at 10,000r / min for 10 min, take the supernatant, and store it in a refrigerator at 4°C in the dark until analysis and determination;
[0052] (2) Sample determination: The operation was the same as in Example 1. The tetracycline was calculated from the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com