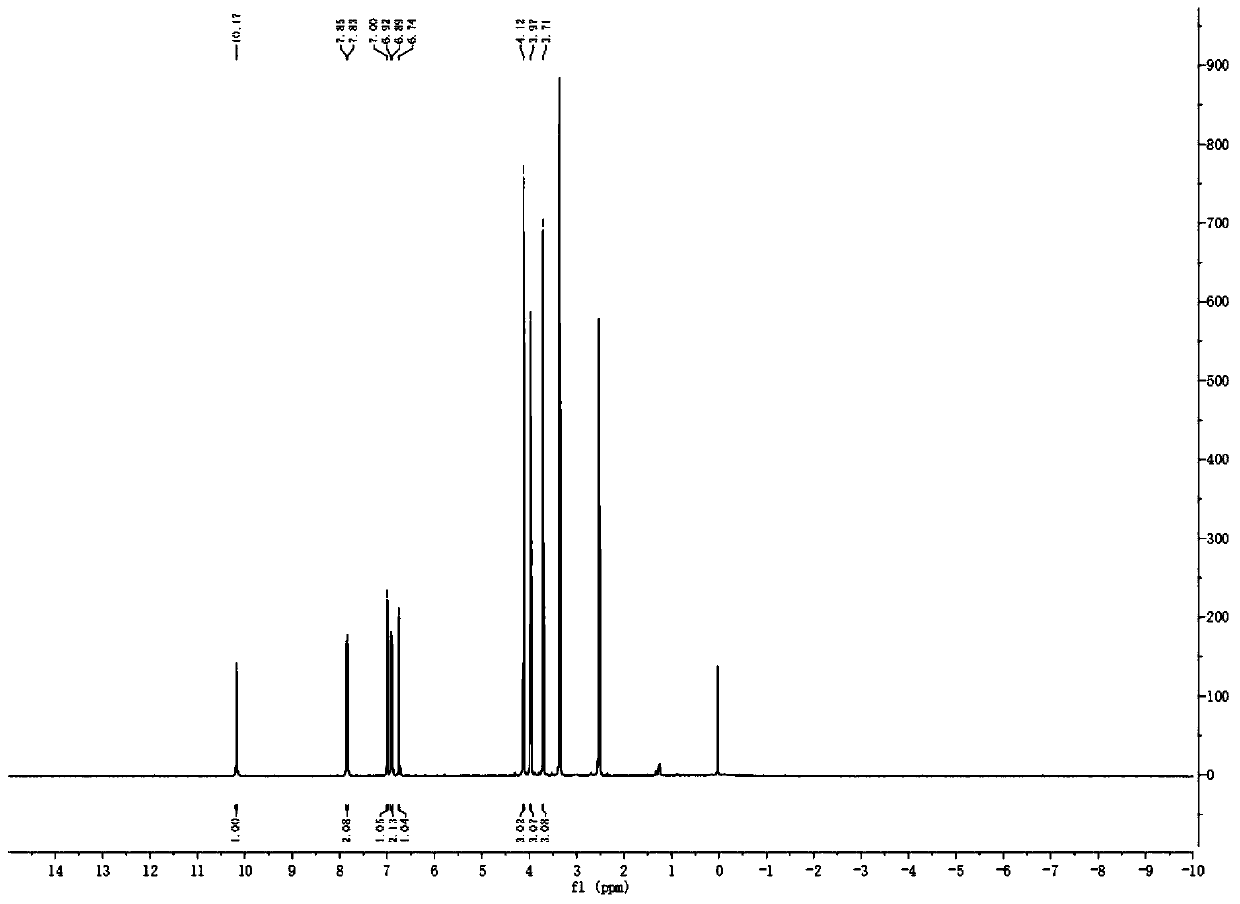
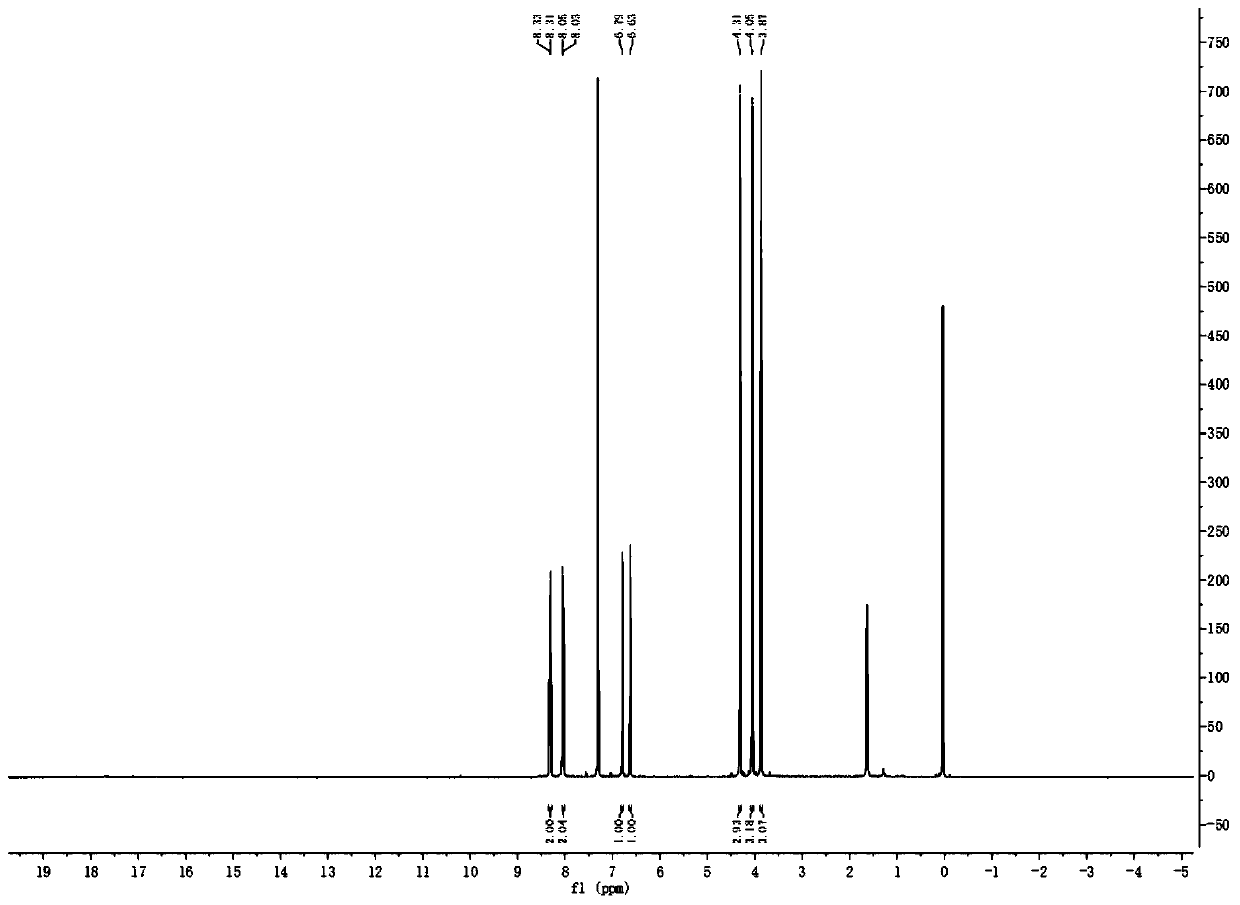
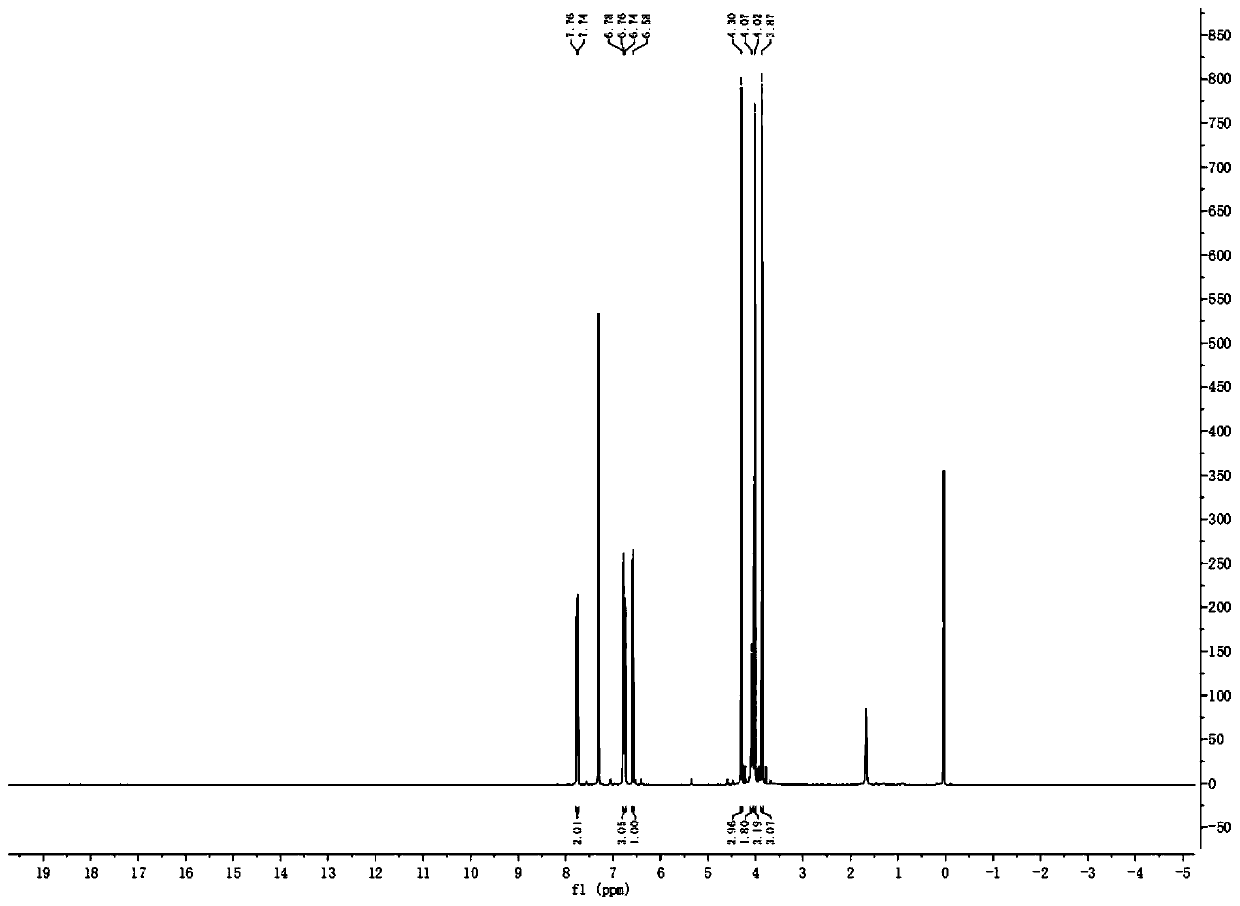
Method used for synthesis of 6-hydroxyl-2, 3, 4-triethoxy-alpha-chloroacetophenone
A technology of chlorinated acetophenone and trimethoxyphenol, applied in the direction of condensation preparation of carbonyl compounds, organic chemistry, etc., can solve the problems of unindustrialized large-scale production, corrosive equipment, difficult to control, etc., and achieve cost saving, simple raw materials, The effect of simple post-processing methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The influence of embodiment 1 different reaction temperature on reaction yield
[0038] Because in the prior art, when carrying out Friedel-Crafts acylation reaction, common reaction system is to use aluminum chloride as catalyst, with nitrobenzene as reaction solvent, therefore, the applicant adopts this reaction system, first investigated different temperature for influence on the yield of the reaction. The specific operation is as follows:
[0039] Weigh (10mmol, 1.84g) 3,4,5-trimethoxyphenol and dissolve it in (20ml) nitrobenzene, slowly add (10mmol, 1.13g) chloroacetyl chloride dropwise in the constant pressure dropping funnel, dropwise Finally, rinse the dropping funnel with 5ml of nitrobenzene, drop the remaining chloroacetyl chloride on the wall into the reaction flask, then add (10mmol, 1.33g) aluminum chloride anhydrous, install a condensation reflux and drying device, Stir and heat the reaction in oil baths at different temperatures, and detect the reaction...
Embodiment 2
[0042] The influence of embodiment 2 different solvents on reaction
[0043] Weigh (10mmol, 1.84g) 3,4,5-trimethoxyphenol and dissolve it in (20ml) different reaction solvents, slowly add (10mmol, 1.13g) chloroacetyl chloride dropwise in the constant pressure dropping funnel, dropwise Finally, rinse the dropping funnel with 5ml of different reaction solvents, drop the remaining chloroacetyl chloride on the wall into the reaction flask, then add (10mmol, 1.33g) anhydrous aluminum chloride, install a condensing reflux and drying device, and adjust the oil bath The pot controls the temperature of the reaction solution at 40° C. and heats the reaction with airtight stirring. The reaction is detected by TLC. After the reaction is completed, 6-hydroxy-2,3,4-trimethoxy-α-chloroacetophenone is obtained after post-processing and purification. Yield (based on 3,4,5-trimethoxyphenol) is as follows:
[0044] solvent Nitrobenzene Dichloromethane acetone Yield / % 62.1 ...
Embodiment 3
[0047] The influence of embodiment 3 different acylating reagents on reaction
[0048] Weigh (10mmol, 1.84g) 3,4,5-trimethoxyphenol and dissolve it in (20ml) dichloromethane, slowly add (10mmol) different acylating reagents dropwise in the constant pressure dropping funnel, and use Rinse the dropping funnel with 5ml of dichloromethane, drop the residual acylating reagent on the wall into the reaction flask, then add (10mmol, 1.33g) aluminum chloride anhydrous, install a condensation reflux and drying device, adjust the oil bath to The temperature of the reaction solution was controlled at 40°C and the reaction was heated with airtight stirring, and the reaction was detected by TLC. After the reaction was completed, the reaction solution was poured into concentrated hydrochloric acid: ice water (1:1), vigorously stirred, acidolysis and cooling, and the solid was precipitated after standing still. Filter, wash with saturated sodium chloride solution until neutral, and vacuum-dry...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com