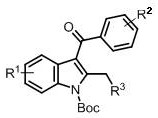
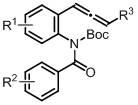
A kind of synthetic method of 3-acylindole compound without metal participation
The technology of acyl indole and synthesis method is applied in the field of synthesis of 3-acyl indole compounds, which can solve the problems of low atom economy and achieve the effects of cheap catalyst, mild reaction temperature and high atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~16
[0023] Embodiments 1~16 A kind of synthetic method of 3-acylindole compound without metal participation:
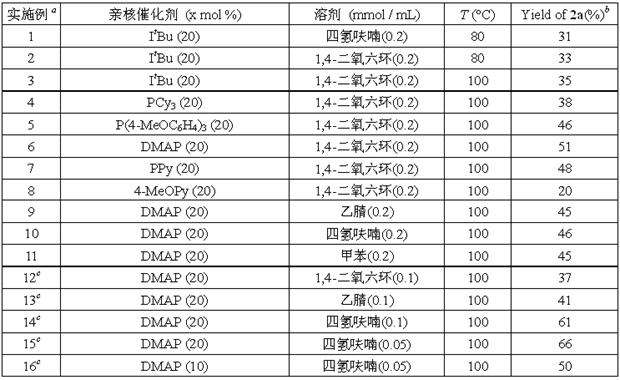
[0024] 0.1 mmol of aniline compound , 0.02 mmol nucleophilic organic catalyst (nucleophilic catalyst) and a stirring magnet were added to the reaction bottle, and then the reaction bottle was brought into a glove box filled with nitrogen, and 0.5~2 mL of anhydrous and oxygen-free solvent ( Solvent). Then, it was sealed and removed from the glove box, and reacted at 100 °C for 48 h. After cooling down to room temperature, it was diluted with an appropriate amount of ethyl acetate and n-dodecane was added as an internal standard. The yield of 3-acylindole compound 2a was detected by high pressure liquid chromatography (HP-GC) and determined by the internal standard. Specific examples are as follows in Table 1:
[0025]
[0026] Table 1
[0027]
[0028] a Unless otherwise specified, the amount of 1a used in all reactions is 0.1 mmol, and the reaction is 20 hour...
Embodiment 17
[0030] Example 17 A method for the synthesis of metal-free 3-acylindole compounds:
[0031] 0.1 mmol of aniline compound , 0.02 mmol 4-dimethylaminopyridine and a stirring magnet were added to the reaction bottle, and then the reaction bottle was brought into a glove box filled with nitrogen, and 2 mL of anhydrous and oxygen-free solvent tetrahydrofuran was added. Then, it was sealed and removed from the glove box, and reacted at 100 °C for 48 h. After cooling down to room temperature, the solvent was distilled off under reduced pressure, and the residue was subjected to column chromatography using a mixed solvent of ethyl acetate-petroleum ether (1 mL: 30 mL) as an eluent to obtain a colorless oily product with a yield of 63%.
[0032] The product is N -tert-butoxycarbonyl-3-benzoyl-2-methylindole, the structural formula is as follows: .
[0033] The result of NMR analysis was 1 H NMR (400 MHz, CDCl 3 ) 1 H NMR (400 MHz, CDCl 3 ) δ 8.13(d, J = 8.4 Hz, 1H), 7.84 (d...
Embodiment 18
[0034] Example 18 A method for the synthesis of metal-free 3-acylindole compounds:
[0035] 0.1 mmol of aniline compound , 0.02 mmol 4-dimethylaminopyridine and a stirring magnet were added to the reaction bottle, and then the reaction bottle was brought into a glove box filled with nitrogen, and 2 mL of anhydrous and oxygen-free solvent tetrahydrofuran was added. Then, it was sealed and removed from the glove box, and reacted at 100 °C for 48 h. After cooling down to room temperature, the solvent was distilled off under reduced pressure, and the residue was subjected to column chromatography using a mixed solvent of ethyl acetate-petroleum ether (1 mL: 40 mL) as the eluent to obtain a colorless oily product with a yield of 59%.
[0036] The product is N -tert-butoxycarbonyl-3-benzoyl-2,5-dimethylindole, the structural formula is as follows: .
[0037] The result of NMR analysis was 1 H NMR (400 MHz, CDCl 3 ) δ 7.98 (d, J = 8.4 Hz, 1H), 7.84 (d, J = 7.2 Hz, 2H), 7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com