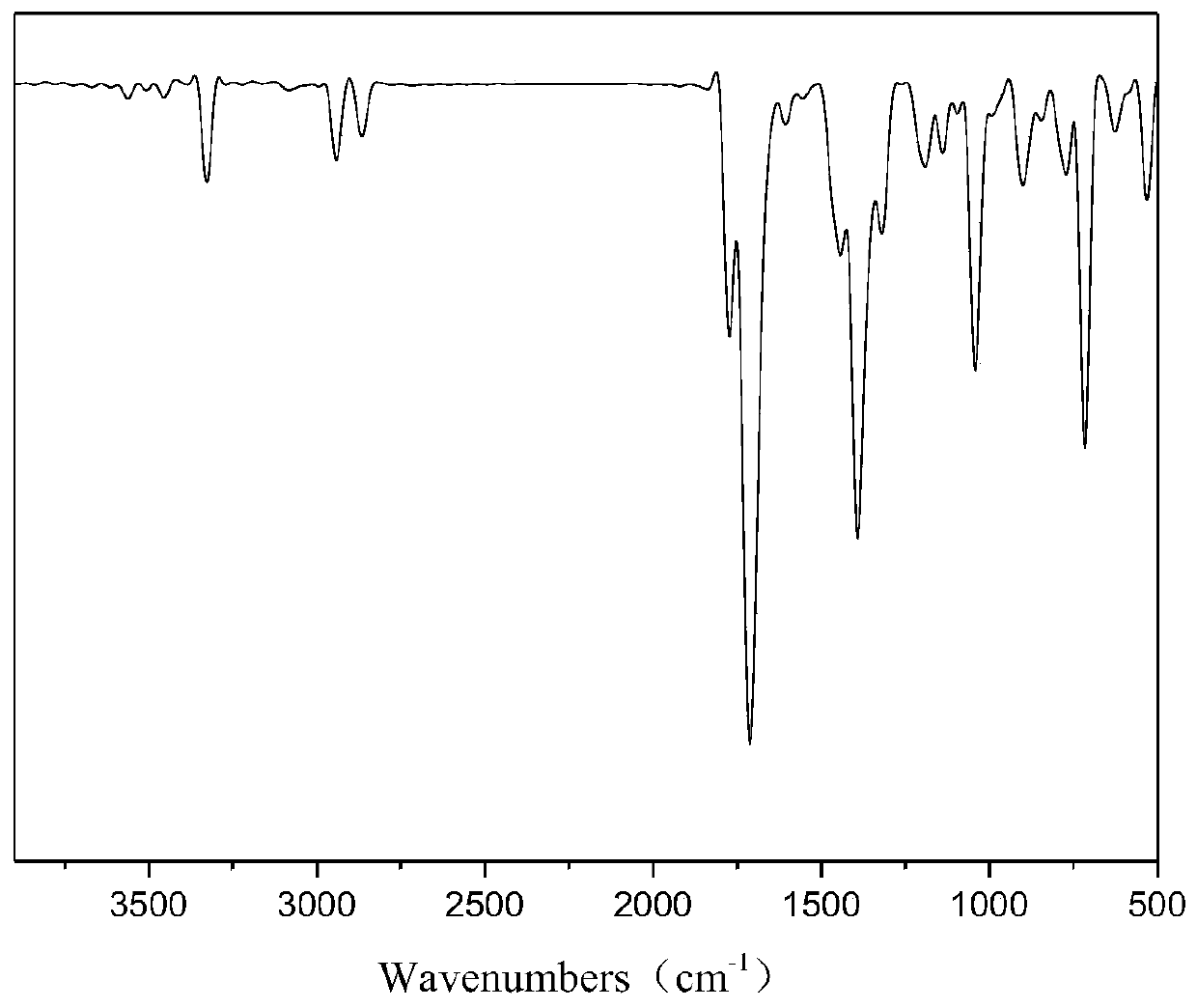
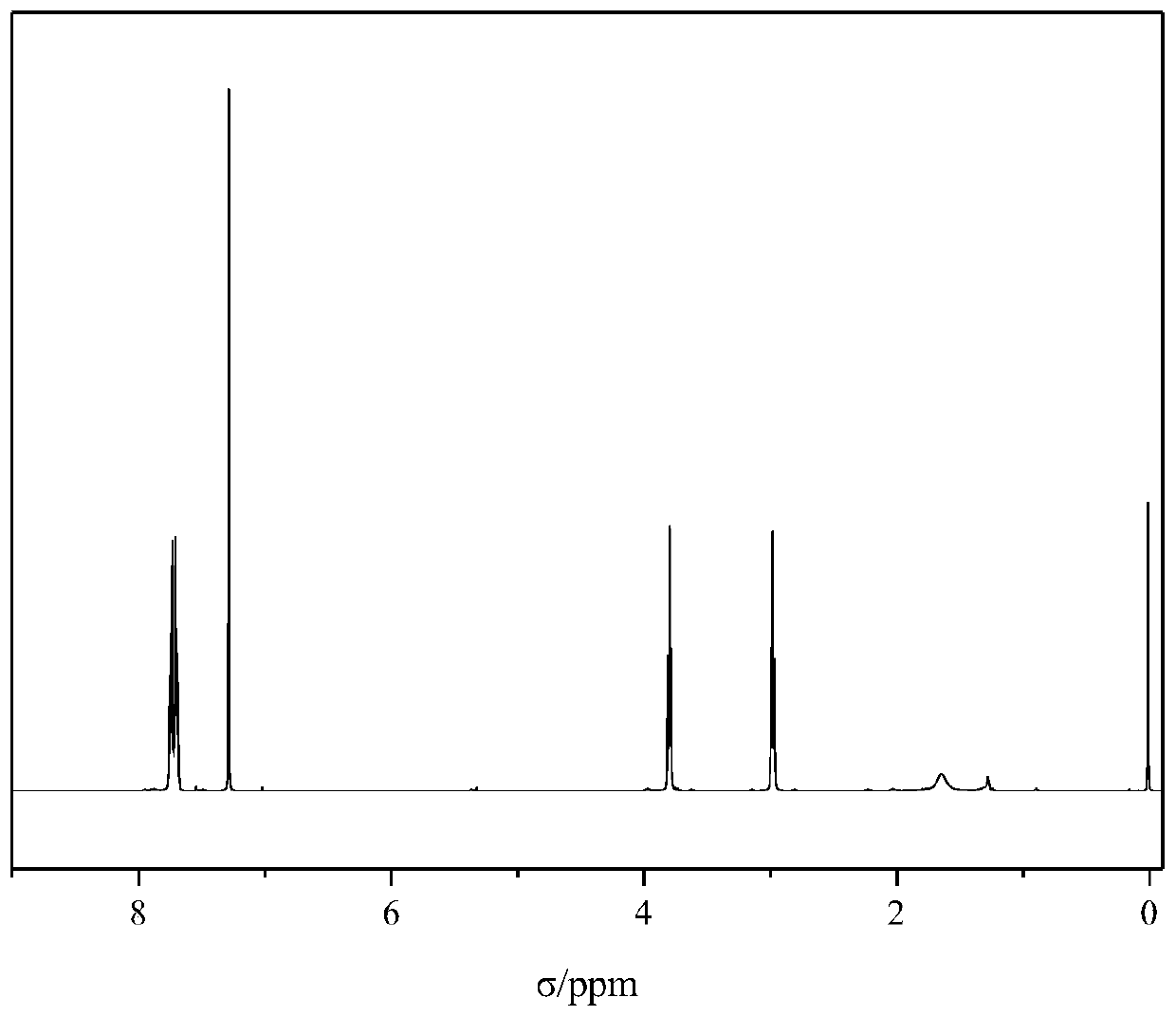
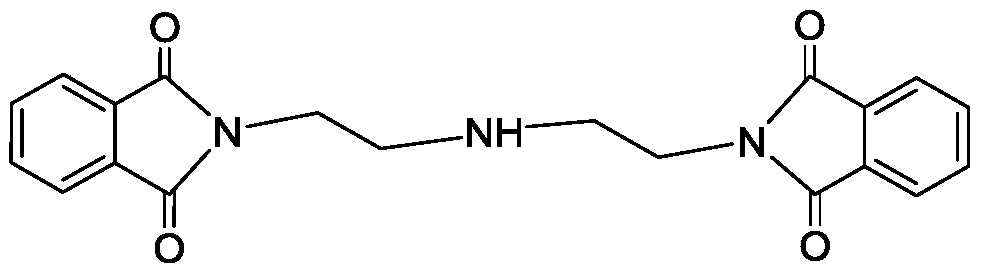
Bis(2-phthalimide)amine and preparation method thereof
A technology of phthalimide and phthalic anhydride, which is applied in the field of diamine and its preparation, can solve the problems of inapplicability to mass production, low product purity, complicated post-treatment, etc., and achieve low cost and high product purity. High, easy post-processing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] The preparation method of the bis(2-phthalimide) amine is obtained through an electrophilic substitution-elimination reaction between phthalic anhydride and diethylenetriamine, wherein glacial acetic acid is used as a solvent, which can act as a proton Catalysis and low cost, using toluene as a water-carrying agent, so that the water generated in the reaction can be quickly separated through ring closure, so that the reaction moves forward and the yield of the product is improved.
[0029] The principle of electrophilic substitution-elimination is as follows,
[0030]
[0031] The preparation method of two (2-phthalimide) amines of the present invention specifically comprises the following steps,
[0032] Step 1, place a three-necked flask equipped with a stirrer in an oil bath, add 2.43-3.86g of diethylenetriamine, 9.27-12.95g of phthalic anhydride, and 40-60ml of glacial acetic acid into the three-necked flask, and then heat from room temperature to 90-100°C, at w...
Embodiment 1
[0039] The preparation method of two (2-phthalimide) amines of the present invention specifically comprises the following steps,
[0040] Step 1, place a three-necked flask equipped with a stirrer in an oil bath, add 2.78g of diethylenetriamine, 9.27g of phthalic anhydride, and 40ml of glacial acetic acid into the three-necked flask, and then heat it from room temperature to 90°C. carry out the ring-opening reaction;
[0041] Step 2, take 20ml of toluene and put it into the dropping funnel to prepare for dropping;
[0042] Step 3, in the case of constant heating, add toluene dropwise while stirring, and the drop can be completed within 30 minutes. The specific rate does not affect the progress of the reaction, and the temperature is raised to 115°C. The higher the temperature, the more conducive to ring closure, and continue the reaction for 4 hours;
[0043] Step 4, after the reaction, cool to room temperature, the mixed system obtained at this time is yellow, and the mixed ...
Embodiment 2
[0049] The preparation method of two (2-phthalimide) amines of the present invention specifically comprises the following steps,
[0050] Step 1, add 2.60g of diethylenetriamine, 10.08g of phthalic anhydride, and 50ml of glacial acetic acid into a three-necked flask, then heat from room temperature to 97°C;
[0051] Step 2, take 22ml of toluene and put it into the dropping funnel and prepare to add it dropwise;
[0052] Step 3, under the condition of constant heating, add toluene dropwise while stirring, and finish dropping after 30 minutes, then raise the temperature to 118°C, the higher the temperature, the better the ring closure, and continue the reaction for 2 hours;
[0053] Step 4, after the reaction, cool to room temperature, the mixed system obtained at this time is yellow, and the mixed system is rotary evaporated at 65°C until no liquid flows out, that is, the solvent glacial acetic acid is removed, and a dark yellow viscous mixed system is obtained ;
[0054] Ste...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com