Use of hemoglobin and hemoglobin derivatives in preparing injection preparation for improving immune function
A technology of hemoglobin and pharmaceutical preparations, applied in the field of biomedicine, can solve problems such as damage to the nervous system, digestive system, and mental confusion of patients, and achieve the effect of improving immune function, high safety, and small dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
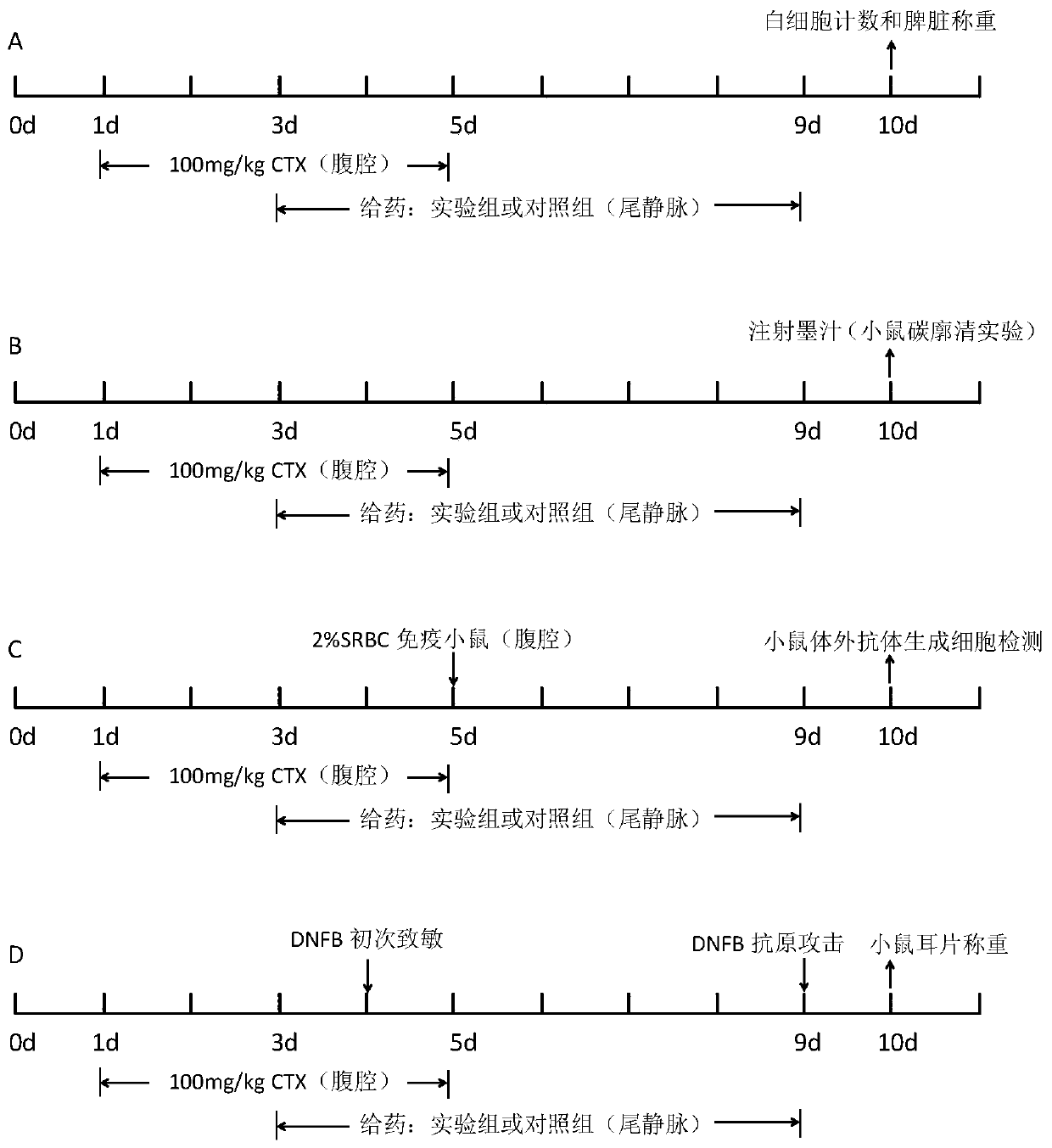
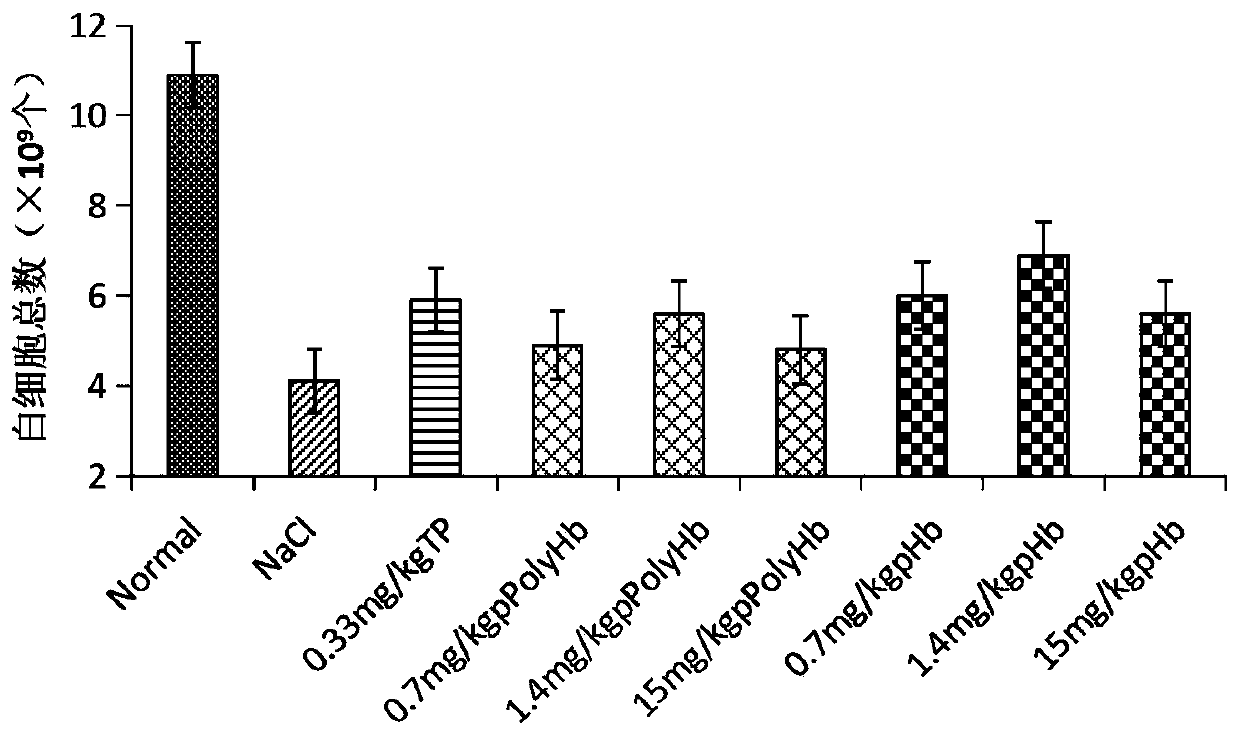
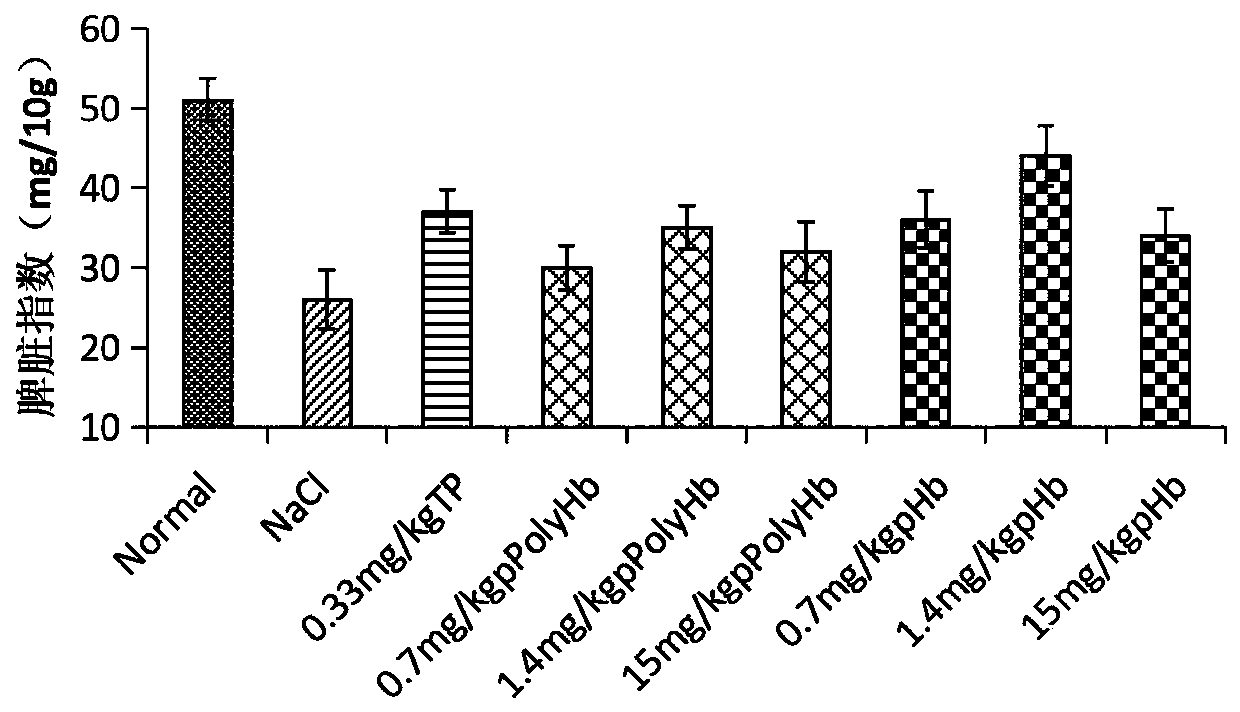
[0051] In the experiment, firstly, 100 mg / kg cyclophosphamide (CTX) was injected intraperitoneally into ICR mice for 5 consecutive days to establish an immunocompromised (immunosuppressed) mouse model. On the third day of modeling, porcine hemoglobin (Hb) or porcine hemoglobin (Hb) or Polyporcine hemoglobin (pPolyHb) was continuously injected for 7 days; in the same way, a positive control group with thymopentin (TP) injection solution (0.33mg / kg), a negative control group with equal volume of normal saline, and no treatment were given every day. An equal volume of normal saline was used as the normal control group. The timeline of mouse modeling, drug injection and sampling points is shown in figure 1 shown. After the test is completed, the white blood cell count, immune-related factors, spleen index measurement, carbon clearance test and other detections are carried out respectively.
[0052] test results:
[0053] 1.1 Effect of porcine Hb and pPolyHb on the total number ...
Embodiment 2
[0066] In the experiment, the Balb / C mouse model of radiation injury and immunocompromise was first established (the whole body of the Balb / C mouse was irradiated 15 minutes away from the qinlon ray for 5 consecutive days), and bovine hemoglobin (0.07mg / kg ), bovine methemoglobin (0.5mg / kg), human carboxyhemoglobin (0.5mg / kg), canine deoxyhemoglobin (2mg / kg), double aspirin cross-linked sheep hemoglobin (16mg / kg), chicken sulphated hemoglobin (15mg / kg) kg) and polymerized bovine hemoglobin mixture (15mg / kg), injected continuously for 7 days; in the same way, a positive control group thymopentin (TP) injection solution (0.33mg / kg), a negative control group with equal volume of normal saline, and no After any treatment, an equal volume of normal saline was given every day as a normal control group. After the test was completed, the immune-related factors and spleen index were measured respectively.
[0067] test results:
[0068] 2.1 Effects of different hemoglobin derivatives...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com