Preparation method of phosphate ester or phosphite ester, electrolyte and secondary battery
A phosphite and phosphoric acid ester technology, applied in secondary batteries, chemical instruments and methods, circuits, etc., can solve the problems of high reaction temperature, high cost, and high raw material cost, and achieve a mild synthesis method, production cost control, and applicability wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
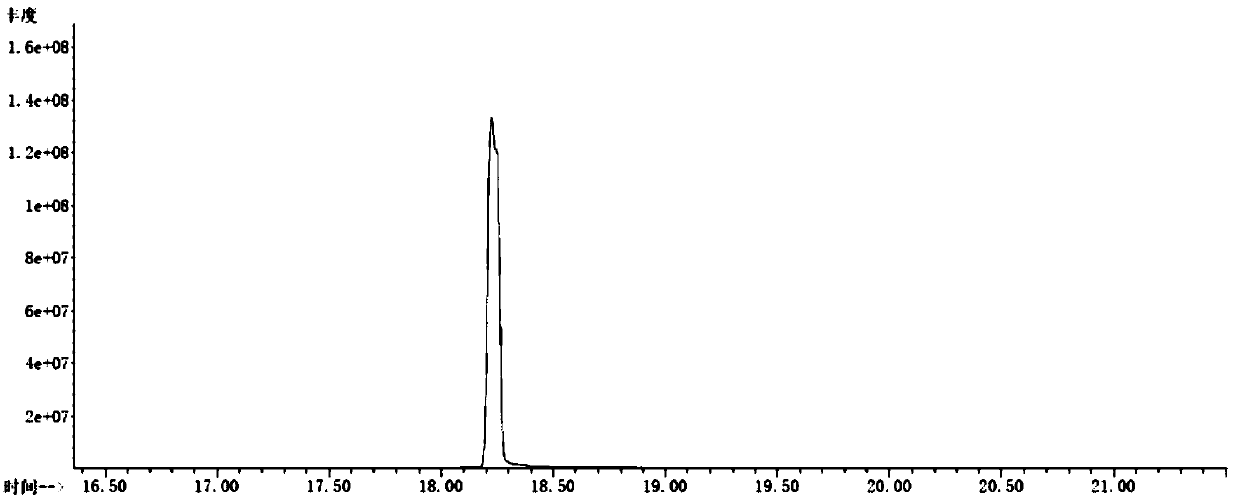
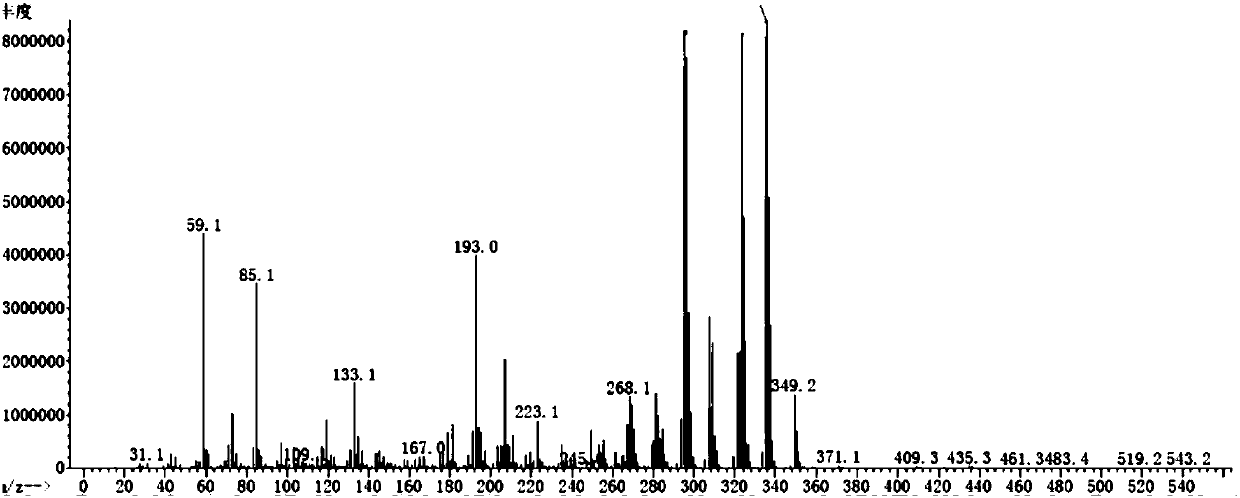
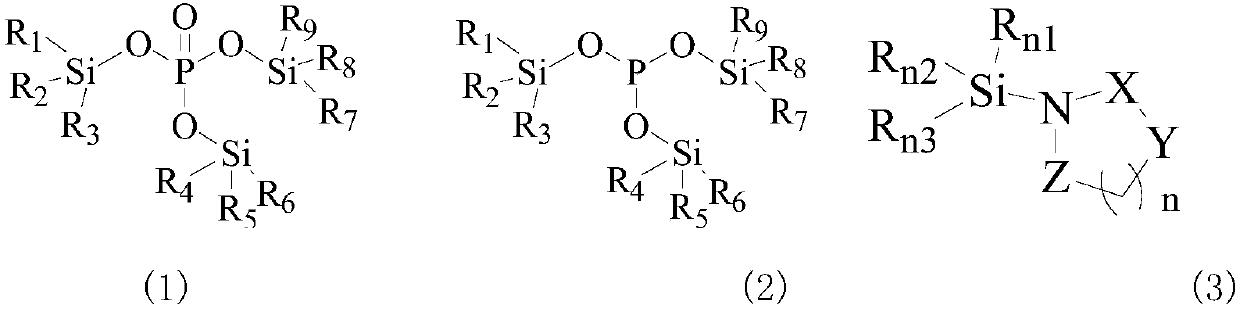
Image
Examples
Embodiment 1
[0076] Weigh 0.26 mol of 2-pyrrolidone, add 0.26 mol of trimethylsilyl chloride and DMF, react at 50°C for 2 hours, and distill under reduced pressure to obtain 3-trimethylsilylpyrrolidone (0.25 mol).
[0077] Weigh 0.05 mol of lithium dihydrogen phosphate, add 0.1 mol of 3-trimethylsilylpyrrolone, and react at room temperature for 1 h. Distillation under reduced pressure (20 mmHg, 72° C.) yielded 0.029 mol of colorless transparent liquid tris(trimethylsilyl) phosphate, with a yield of 86% and a purity of 99%.
Embodiment 2
[0079] Weigh 0.05mol phosphorous acid, add 0.15mol 3-trimethylsilylpyrrolidone, dissolve in THF, and react at 70°C for 4h. The pyrrolidone solid was obtained by filtration, washed three times with 30 ml of petroleum ether, the petroleum ether phase was combined, the solvent was removed, and the GC-MS test was performed. Distilled under reduced pressure to obtain 9 g, a colorless transparent liquid. Then add sodium metal, react under nitrogen protection at 140°C-150°C for 20h under anhydrous conditions, and then distill under reduced pressure after cooling to obtain 0.13mol of tris(trimethylsilyl)phosphite (20mmHg, 75°C).
Embodiment 3
[0081] Weigh 0.26 mol of pyrrolidine, add 0.26 mol of dimethylvinylchlorosilane and DMSO, react at 50° C. for 10 h, and distill under reduced pressure to obtain 1-(dimethylvinylsilyl)pyrrolidine (0.25 mol).
[0082]Weigh 0.05 mol of sodium dihydrogen phosphate, add 1-(dimethylvinylsilyl)pyrrolidine (0.25 mol) and react at room temperature for 1 h. Distillation under reduced pressure (20 mmHg, 72° C.) yielded 0.045 mol of tris(dimethylvinylsilyl)phosphate as a colorless transparent liquid with a yield of 90% and a purity of 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com