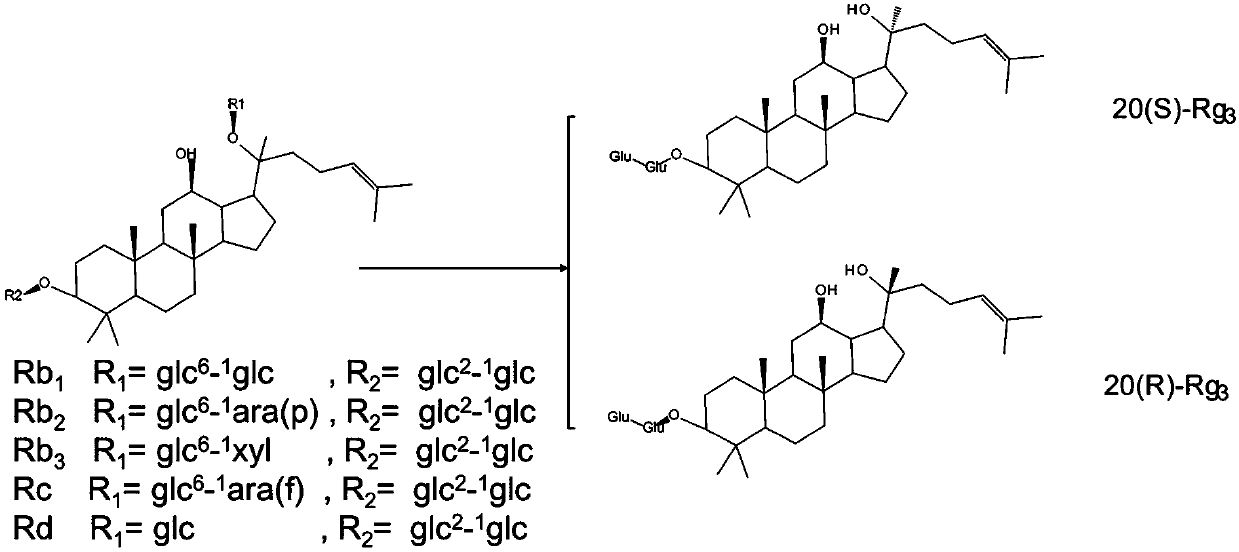
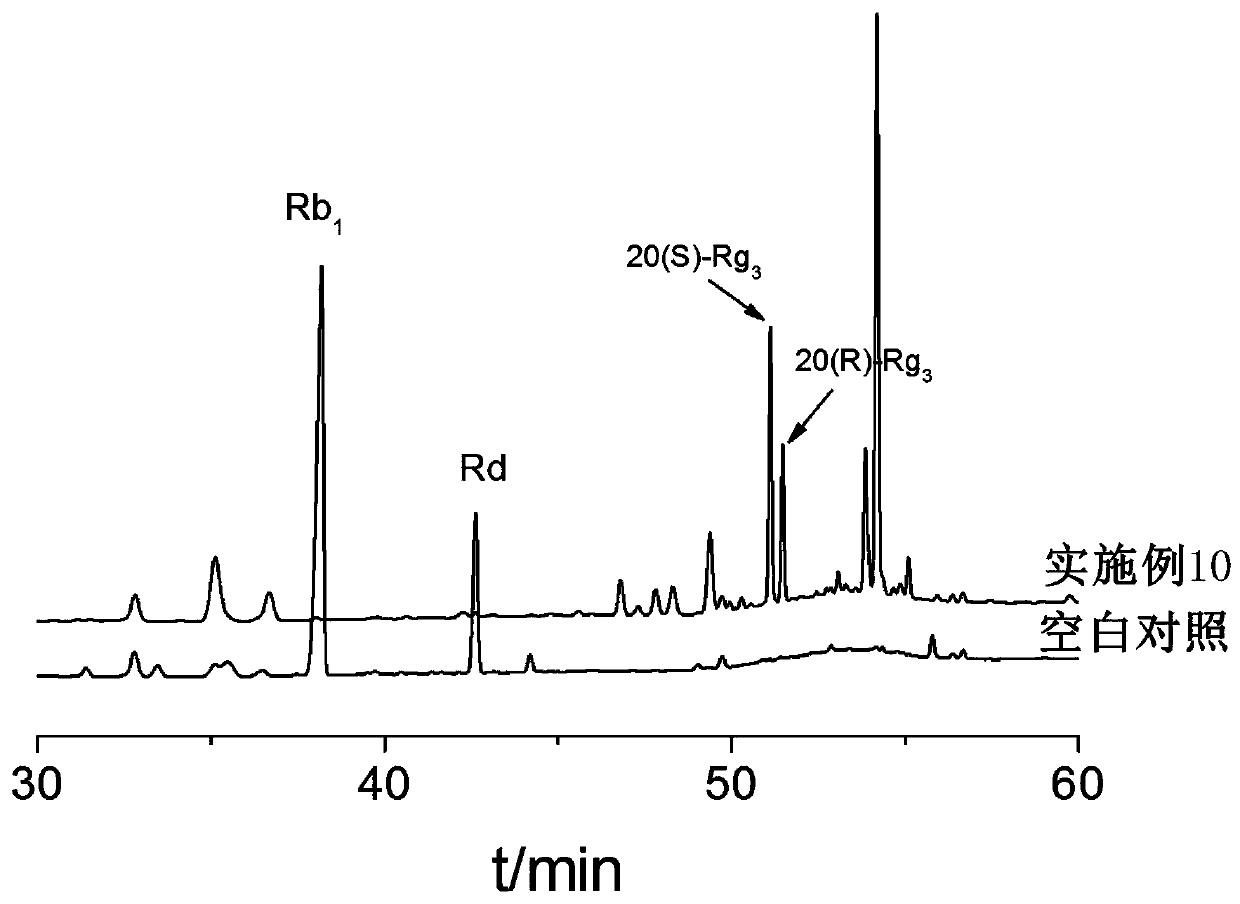
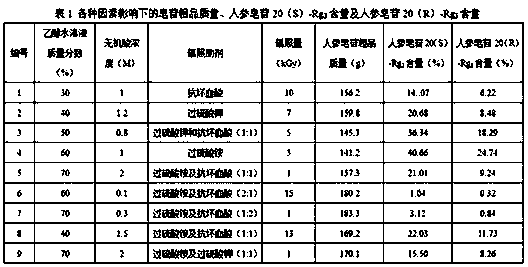
Method for preparing ginsenoside Rg3 by adopting irradiation
A ginsenoside and irradiation technology, applied in chemical instruments and methods, glycoside steroids, steroids, etc., can solve the problems of low yield of Rg3, dehydration of panaxadiol group saponins, and limited industrial application, and achieve selective hydrolysis The effect of thorough, short catalytic time and complete selective hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] A kind of preparation ginsenoside Rg by irradiation 3 method, including the following steps:
[0049] A. Taking ginsengdiol group saponin as raw material, adding the aqueous ethanol solution of inorganic acid to obtain the ethanol solution of ginsengdiol group saponin-inorganic acid with a mass volume ratio of 1:5;
[0050] B. adding mass ratio to the ethanol aqueous solution of ginseng diol group saponin-inorganic acid is 0.5% irradiation auxiliary agent, stirs until completely dissolving;
[0051] C. the mixed solution obtained through step B is placed in the irradiation reactor, sealed, to 60 Co-γ ray is the irradiation source, with 0.5kGy / h as the irradiation dose rate, and irradiating 1kGy at room temperature; after purification by the adsorption column, the target ginsenoside Rg is obtained after detection. 3 Crude.
[0052] The irradiation reactor is made of glass or acid-proof and radiation-resistant plastic, and its volume can be set freely according to actu...
Embodiment 2
[0054] A kind of preparation ginsenoside Rg by irradiation 3 method, including the following steps:
[0055] A. Taking ginsengdiol group saponin as raw material, adding the aqueous ethanol solution of mineral acid, obtaining the ethanol solution of ginsengdiol group saponin-mineral acid with a mass volume ratio of 1:10;
[0056] B. in the ethanol aqueous solution of panaxadiol group saponin-inorganic acid, adding mass ratio is the irradiation aid of 5%, stirs until fully dissolving;
[0057] C. the mixed solution obtained through step B is placed in the irradiation reactor, sealed, to 60 Co-γ ray is the irradiation source, with 3kGy / h as the irradiation dose rate, and irradiating 15kGy at room temperature; after purification by the adsorption column, the target ginsenoside Rg is obtained after detection. 3 Crude.
Embodiment 3
[0059] A kind of preparation ginsenoside Rg by irradiation 3 method, including the following steps:
[0060] A. Take ginsengdiol saponins as raw material, add the ethanol aqueous solution of mineral acid, obtain the ethanol aqueous solution of the ginseng diol group saponin-mineral acid of mass volume ratio 1:7;
[0061] B. in the ethanol aqueous solution of panaxadiol group saponin-inorganic acid, adding mass ratio is the radiation aid of 2%, stirs until fully dissolving;
[0062] C. the mixed solution obtained through step B is placed in the irradiation reactor, sealed, to 60 Co-γ ray is used as the irradiation source, with 1.5kGy / h as the irradiation dose rate, 4kGy is irradiated at room temperature; after purification by the adsorption column, the target ginsenoside Rg is obtained after detection. 3 Crude.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com