Process for preparing benzocaine through solvent-free hydrogenation
A benzocaine and solvent-free technology, which is applied in the chemical industry, can solve the problems of excessive waste water, yield impact, and no improvement involved, so as to reduce pollution, reduce the discharge of organic waste liquid, good reaction yield and product purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
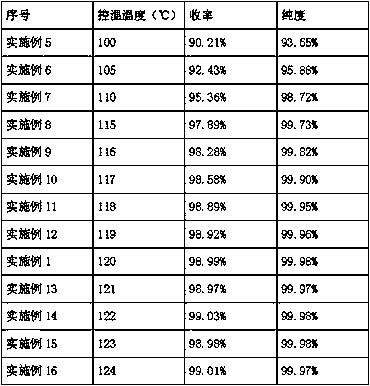
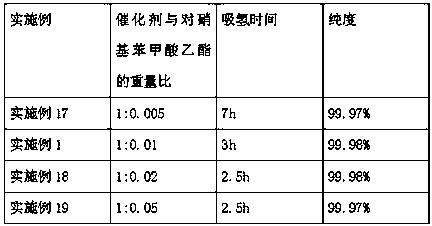
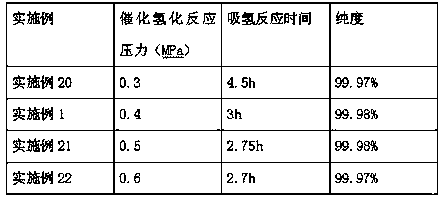
[0022] Add 500g of ethyl p-nitrobenzoate into a 1L autoclave, control the temperature at 120°C, keep it warm for 1h, then add 5g of activated Raney nickel catalyst, replace it with nitrogen three times, then hydrogen three times, and then stir Pass hydrogen to 0.4MPa, and carry out the hydrogenation reaction at a temperature of 120°C. When the pressure no longer drops, after the hydrogenation reaction is completed, keep the temperature for 4 hours, and discharge the hydrogen gas to stop the reaction.
[0023] The GC analysis results of the sampling analysis showed that the purity of benzocaine was 99.98%, ethyl p-nitrobenzoate was not detected, and the rest were unknown components. Filter the material through a precision filter, press into 500g of water for crystallization, stir until the temperature reaches 25°C, centrifuge to obtain a wet product, and vacuum dry for 12 hours to obtain 418.8g of the final product with a yield of 98.99%.
Embodiment 2
[0025] Add 500g of ethyl p-nitrobenzoate into a 1L autoclave, control the temperature at 120°C, keep it warm for 1h, then add 7.5g of activated Raney nickel catalyst, replace it with nitrogen three times, then hydrogen three times, then stir Pass hydrogen down to 0.4MPa, and carry out the hydrogenation reaction at a temperature of 120°C. When the pressure no longer drops, after the hydrogenation reaction ends, keep the temperature for 4 hours, and discharge the hydrogen to stop the reaction.
[0026] The GC analysis results of the sampling analysis showed that the purity of benzocaine was 99.97%, ethyl p-nitrobenzoate was not detected, and the rest were unknown components. Filter the material through a precision filter, press into 500g of water for crystallization, stir until the temperature reaches 25°C, centrifuge to obtain a wet product, and vacuum dry for 12 hours to obtain 419.0g of the final product with a yield of 99.04%.
Embodiment 3
[0027] Embodiment 3,4 is the amplification experiment
[0028] Embodiment 3 (amplification experiment of embodiment 1)
[0029] To scale up the experiment, add 20kg of ethyl p-nitrobenzoate into a 50L autoclave, control the temperature at 120°C, keep it warm for 2 hours, then add 200g of activated Raney nickel catalyst, replace it with nitrogen three times, and then replace it with hydrogen three times. Then pass hydrogen to 0.4MPa under stirring, and carry out hydrogenation reaction at a temperature of 120 ° C. When the pressure no longer drops, after the hydrogenation reaction is completed, keep warm for 4 hours, and discharge hydrogen to stop the reaction.
[0030] The GC analysis results of the sampling analysis showed that the purity of benzocaine was 99.97%, ethyl p-nitrobenzoate was not detected, and the rest were unknown components. Filter the material through a precision filter, press into 20kg of water for crystallization, stir until the temperature reaches 25°C, ce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com