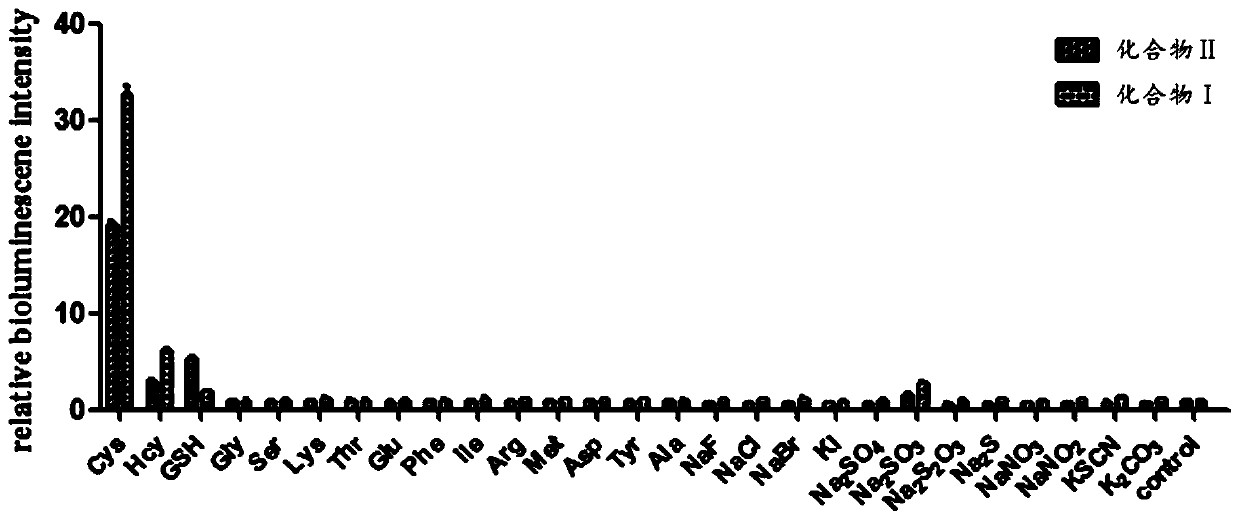
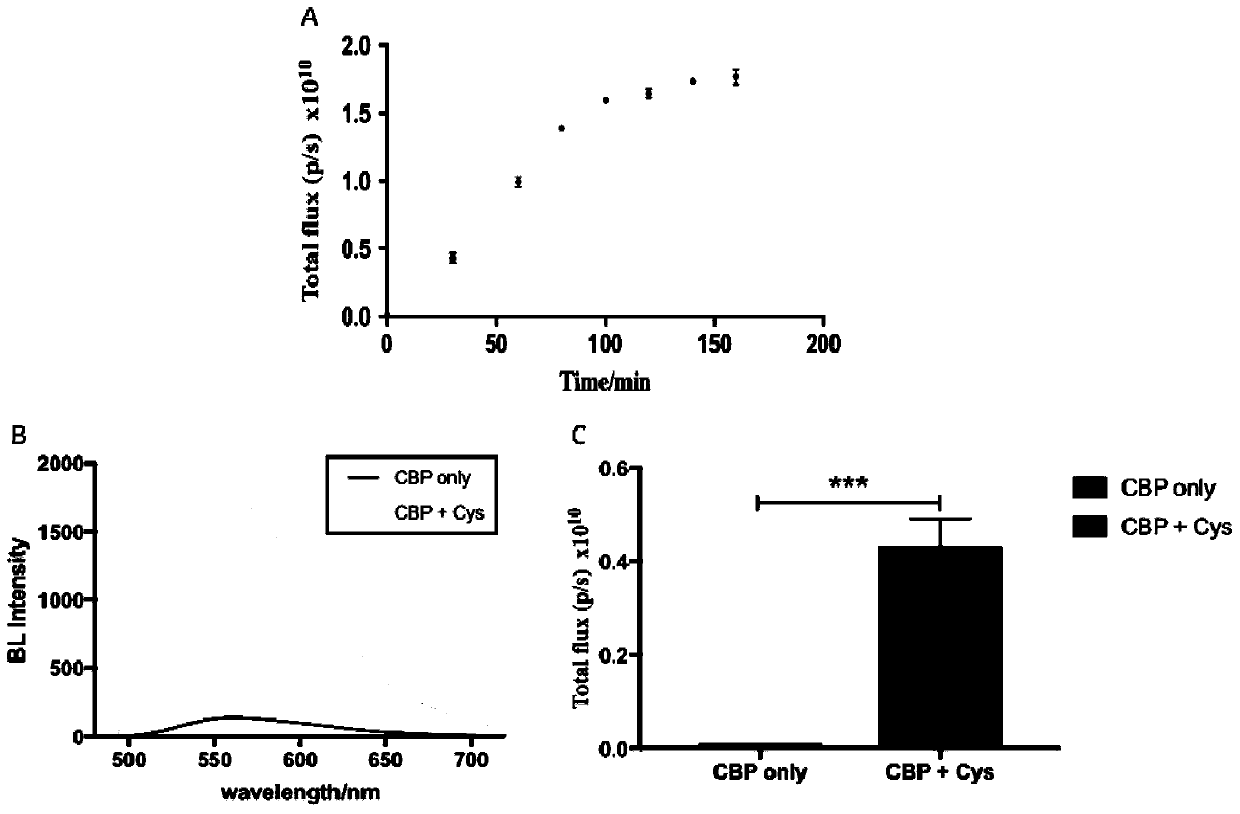
Multi-element heterocyclic compound, preparation method thereof and application of multi-element heterocyclic compound in cysteine detection
A technology of heterocyclic compounds and cysteine, which is applied in the field of multi-component heterocyclic compounds and its preparation and its application in the detection of cysteine. Solve problems such as large organic solvents, achieve high sensitivity, stable bioluminescent signal, and high-sensitivity detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
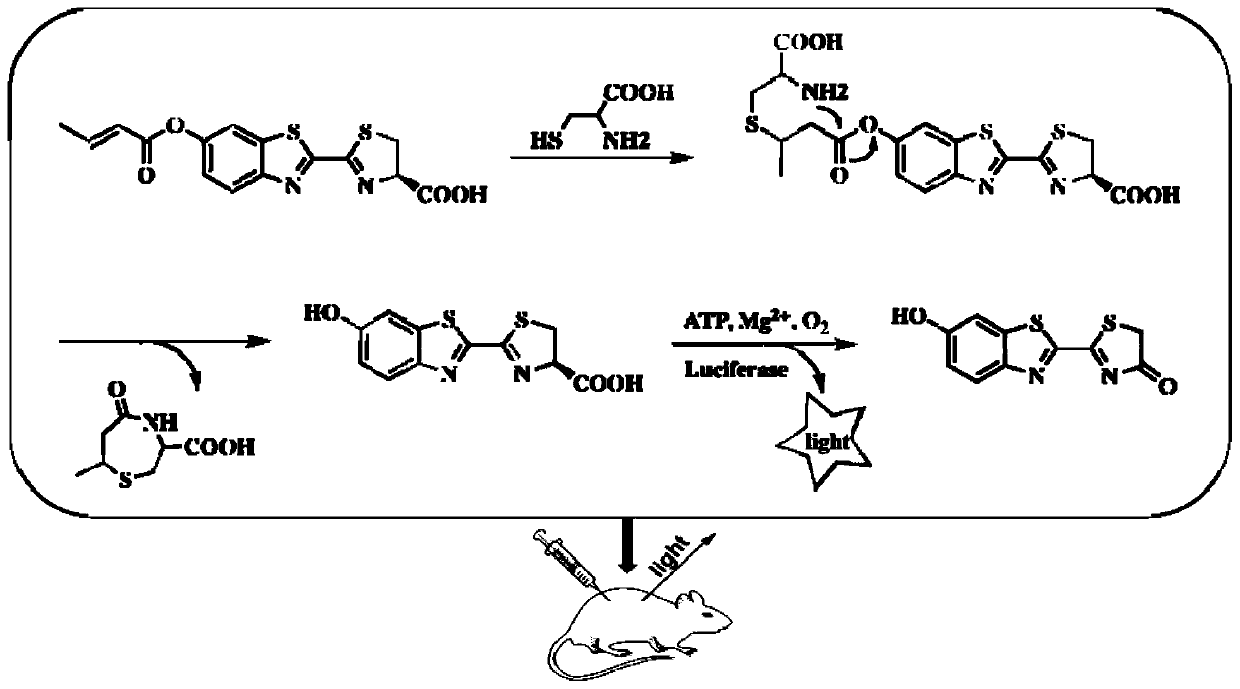
[0051] The synthesis of compound shown in formula I, route is as follows:
[0052]
[0053] Proceed as follows:
[0054] Compound 1 (352mg, 2.0mmol) was placed in a round-bottomed flask, dissolved in 20mL of dichloromethane (DCM), stirred in an ice bath, triethylamine (832uL, 6.0mmol) was added, S-2 (576uL, 6.0mmol ) was dissolved in 10 mL of dichloromethane, added dropwise to the flask, and reacted at room temperature for 5 h. Compound 2 was obtained by column chromatography with a yield of 94.7%. 1 H NMR (400MHz, DMSO) δ8.30 (d, J = 9.0Hz, 1H), 8.21 (d, J = 2.3Hz, 1H), 7.55 (dd, J = 8.9, 2.4Hz, 1H), 7.26–7.14 (m,1H),6.21(dd,J=15.5,1.7Hz,1H),1.98(dd,J=6.9,1.7Hz,3H). 13 C NMR (101MHz, DMSO) δ163.94(s), 150.22(s), 149.36(s), 148.80(s), 137.43(s), 136.28(s), 125.21(s), 123.28(s), 121.01 (s), 115.94(s), 113.25(s), 18.00(s).
[0055] Compound 2 (122 mg, 0.5 mmol) was dissolved with a mixture of 8 mL of dichloromethane and methanol, and D-cysteine hydrochloride dissolved ...
Embodiment 2
[0057] The synthesis of compound shown in formula II, route is as follows:
[0058]
[0059] Proceed as follows:
[0060] Compound 1 (88mg, 0.5mmol) was placed in a round bottom flask, dissolved in 5mL of dichloromethane (DCM), stirred in an ice bath, triethylamine (208uL, 1.5mmol) was added, S-1 (122uL, 1.5mmol ) was dissolved with 5mL of dichloromethane, added dropwise to the flask, and reacted at room temperature for 5h. Obtained by column chromatography (V 石油醚 :V 乙酸乙酯 =10:1~30:1) Solid compound 3, the yield was 94.8%. 1 H NMR (400MHz, DMSO) δ8.32(d, J=9.0Hz, 1H), 8.25(d, J=2.3Hz, 1H), 7.59(dd, J=9.0, 2.4Hz, 1H), 6.61(dd ,J=17.3,1.3Hz,1H),6.48(dd,J=17.3,10.2Hz,1H),6.23(dd,J=10.2,1.3Hz,1H). 13 C NMR (101MHz, DMSO) δ163.94(s), 150.02(s), 149.46(s), 137.57(s), 136.29(s), 134.34(s), 127.23(s), 125.29(s), 123.17 (s), 115.92(s), 113.23(s).
[0061] Compound 3 (115 mg, 0.5 mmol) was dissolved with a mixture of 8 mL of dichloromethane and methanol, and D-cysteine hydroc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com