N-methylpyrrolidine preparation method
A technology of methylpyrrolidine and methylamine, which is applied in the field of preparation of organic bases, can solve the problems of complex process, large investment in production equipment and high production cost of products, and achieves the advantages of simple synthesis process route, reduced equipment investment, and reduced overall cost. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The invention provides a kind of preparation method of N-methylpyrrolidine, comprises the following steps:
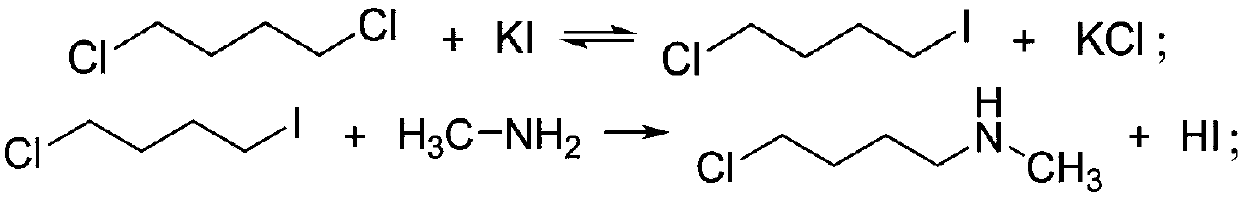
[0025] a) under potassium iodide catalytic conditions, 1,4-dichlorobutane and methylamine aqueous solution are mixed and reacted in an ether solvent to obtain N-methylpyrrolidine;
[0026] The ether solvent can form a hydrogen bond with methylamine, and its boiling point is greater than the azeotropic point of methylamine and water.
[0027] In the preparation method provided by the present invention, N-methylpyrrolidine can be prepared by directly mixing and reacting 1,4-dichlorobutane, methylamine aqueous solution and potassium iodide in an ether solvent. The process specifically includes:
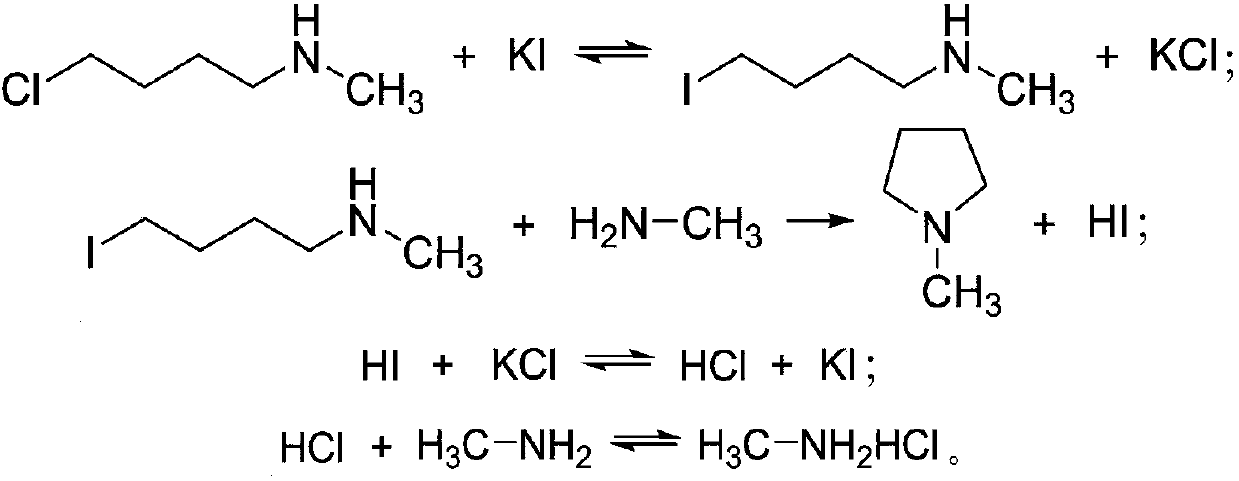
[0028] a1) Under the catalytic conditions of potassium iodide, 1,4-dichlorobutane and methylamine aqueous solution are mixed and reacted in an ether solvent to obtain a reaction solution containing N-methylpyrrolidine and methylamine hydrochloride;
[0029] a2) adding alka...
Embodiment 1
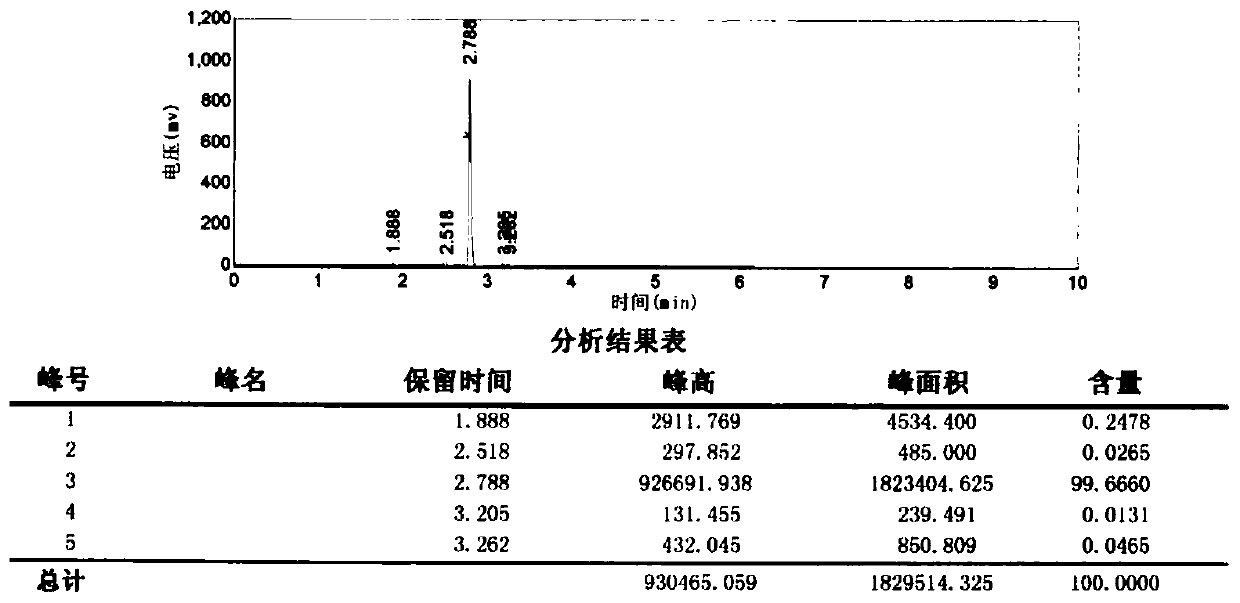
[0045] Into a 1L four-necked glass reaction flask equipped with a mechanical stirrer, a thermometer and a brine-cooled reflux condenser, add 300mL of diglyme, 63.5g (0.5mol) of 1,4-dichlorobutane, 136g (1.75mol) 40wt% methylamine aqueous solution and 2.1g (0.013mol) potassium iodide, the molar ratio of 1,4-dichlorobutane, methylamine and potassium iodide is 1:3.5:0.026. Heating with an electric heating mantle under stirring, raised the temperature to 110°C in 2 hours, and kept the reaction for 4 hours. Then change to a water bath for cooling, lower the temperature to 30°C, and slowly add 10wt% sodium hydroxide aqueous solution at a constant temperature of 30°C to adjust the pH to 12-13. Afterwards, the reflux device was changed to an atmospheric distillation device, and the fraction collected at 50-51°C was the azeotrope of methylamine and water, the fraction at 81-83°C was N-methylpyrrolidine, and the fraction at 101-103°C was water. For vacuum distillation, diglyme is recov...
Embodiment 2
[0047] N-methylpyrrolidine was prepared with reference to the method steps of Example 1, the difference being that the solvent was changed to anisole.
[0048] The results showed that the yield of N-methylpyrrolidine was 88.2%, and the gas chromatography purity was 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com