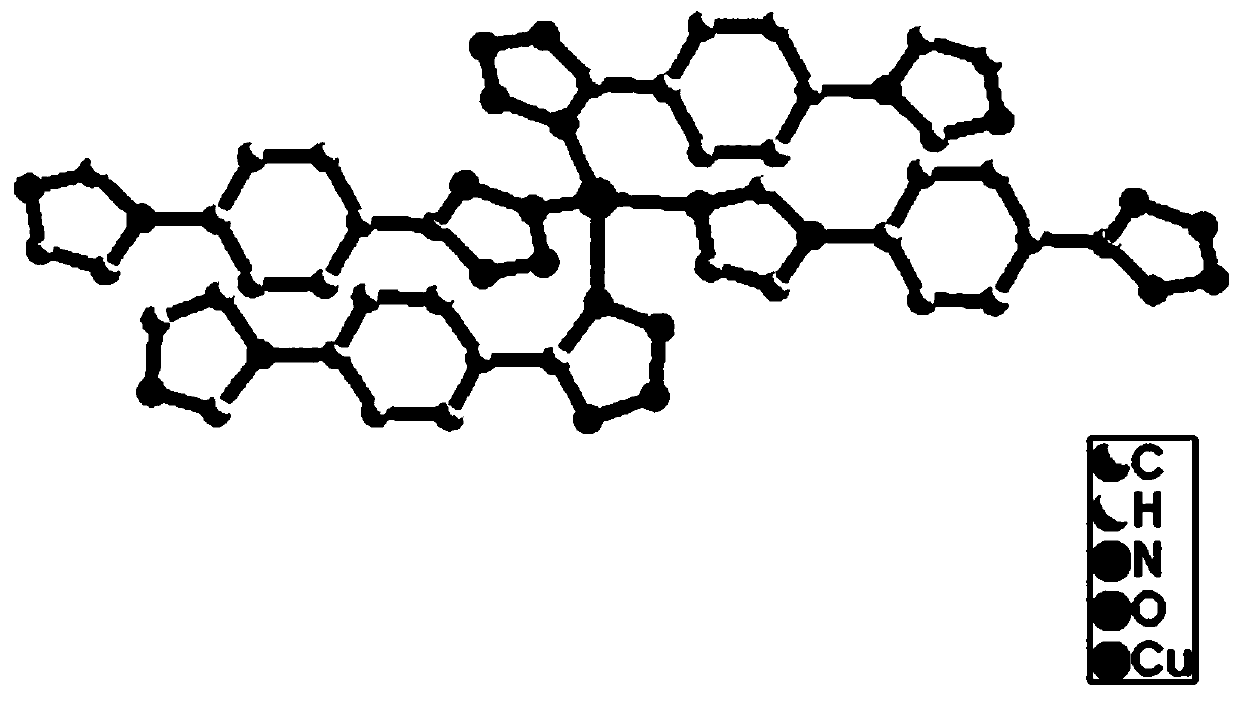
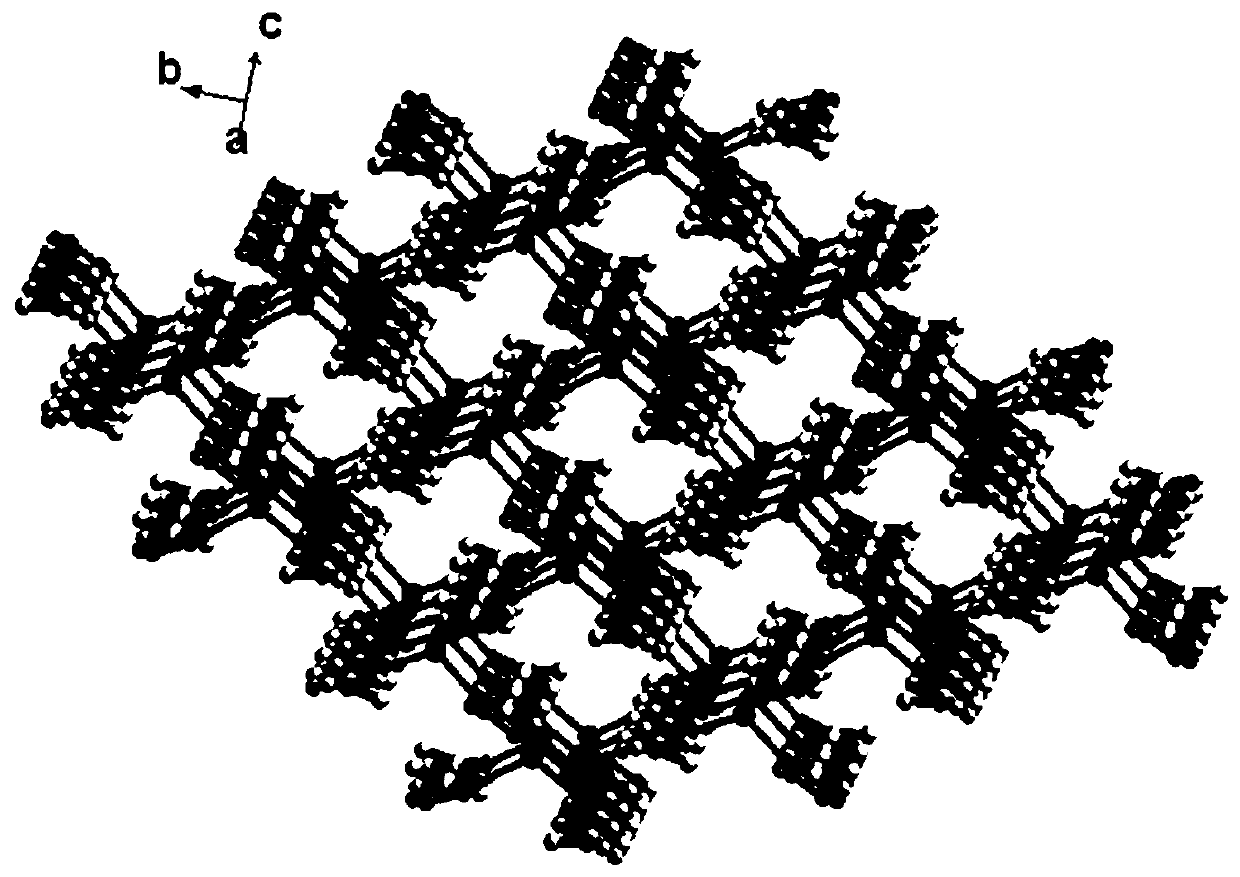
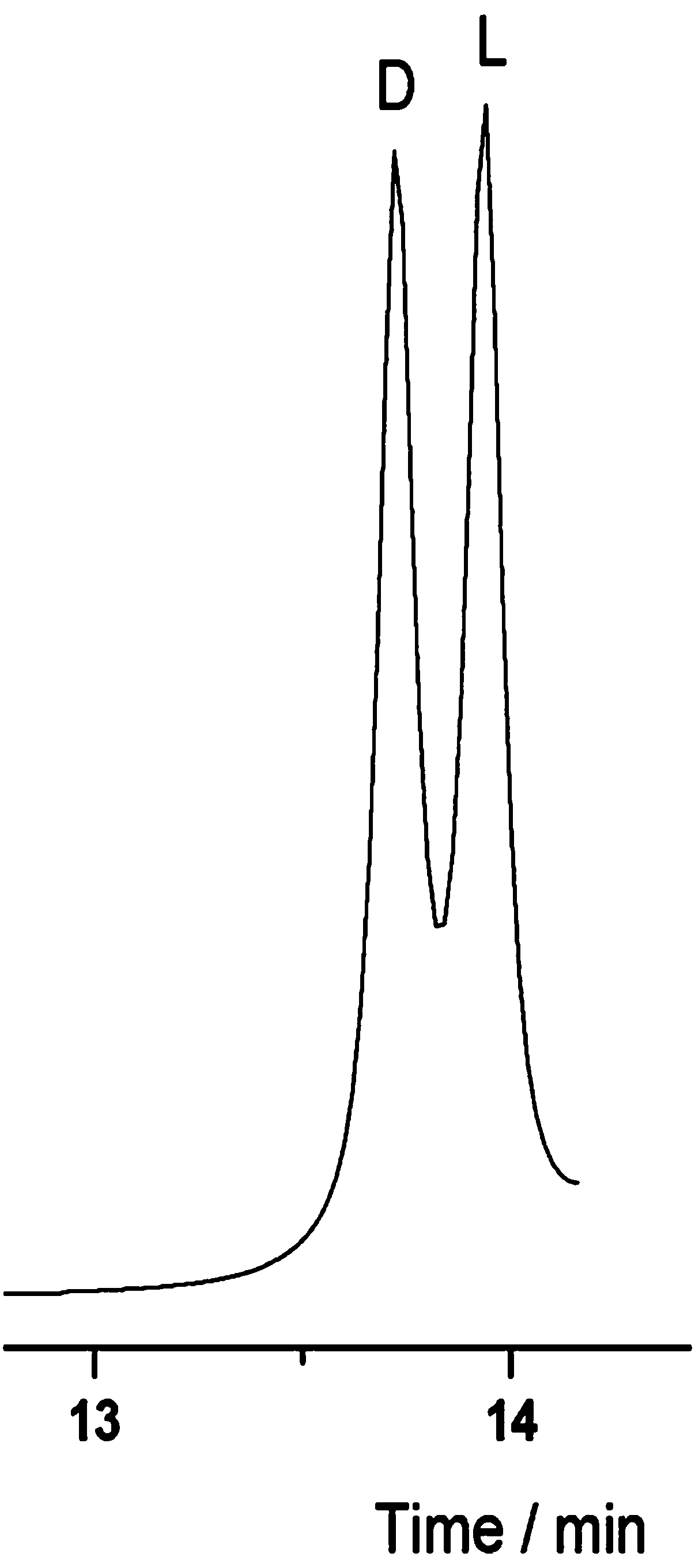
Preparation and application of Cu (I) chiral MOF material based on 5-(4-imidazol-1-phenyl)-1H-tetrazole
A phenyl and chiral technology, applied in the field of preparation of Cu chiral metal-organic framework materials, can solve the problems of insufficient chiral stationary phase materials, affecting the development of chromatographic separation methods, etc., achieving novel structure, strong anti-interference ability, The effect of strong recognition ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Preparation of Cu(I) chiral metal-organic framework based on 5-(4-imidazole-1-phenyl)-1H-tetrazole
[0024] Step 1, HL (0.10 mmol, 21 mg), L-Glu (0.10 mmol, 15 mg) and Cu(NO 3 ) 2 ·6H 2 The mixture of O (0.50mmol, 15 mg) was dissolved in 2 mL of DMF (N,N-dimethylformamide) and put into a vial, and then ultrasonicated in a sonicator to completely dissolve it to obtain solution 1;
[0025] Step 2. Add the newly prepared NaOH (1mol / L) solution dropwise to solution 1 obtained in step 1. When adding 0.02 mL in total, the pH value of the solution is 9 to obtain solution 2;
[0026] Step 3: Place the solution 2 obtained in Step 2 in an oven at 100 °C for 48 h to obtain a red bulk single crystal, which is washed with ethanol and dried for later use. The yield is 30%.
Embodiment 2
[0027] Embodiment 2: X-ray single crystal diffraction analysis
[0028] X-ray single crystal diffraction was tested on a Bruker APEX II CCD diffractometer at a test temperature of 293(2) K. During the test, the graphite monochromator of the instrument was used to monochromatize the Mo-Kα ray (wavelength λ=0.71073Å) to determine the unit cell parameters and collect data using the ω-scan technique. The integration of the diffraction lines was done by the SAINT program, followed by the semi-empirical absorption correction by the SADABS program. The analysis of the crystal structure was completed by the SHELXS program in the SHELXTL software package, and refined by the SHELXL program after being solved by the direct method. The position of the metal atom is determined by the E-map of the direct method, and other non-hydrogen atoms are determined by the difference Fourier function method and the least square method, and then anisotropic refinement is performed according to the the...
Embodiment 3
[0030] Embodiment 3: chiral resolution experiment
[0031] Apply the Cu(I) chiral MOF material prepared in Example 1 on a capillary column, and use a gas chromatograph (GC-2014C) to perform a chiral resolution test. The specific operations are as follows:
[0032] (1) Prepare the Cu(I) chiral MOF material prepared in Example 1 into a solution. The specific operation is: take the stationary phase of the required mass and place it in a clean volumetric flask, and add the required volume of solvent, After it is completely dissolved, it will be degassed by ultrasonic, and then it will be made into a solution with the required concentration;
[0033] (2) use N 2 Press the solution evenly into about 1 / 3 of the pretreated capillary column at a speed of about 1 cm / s, pull the end of the capillary immersed in the solution out of the liquid surface, and use the same N 2 The flow rate pushed the liquid column out of the column, and continued to blow for 4 h, then programmed the tempera...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com