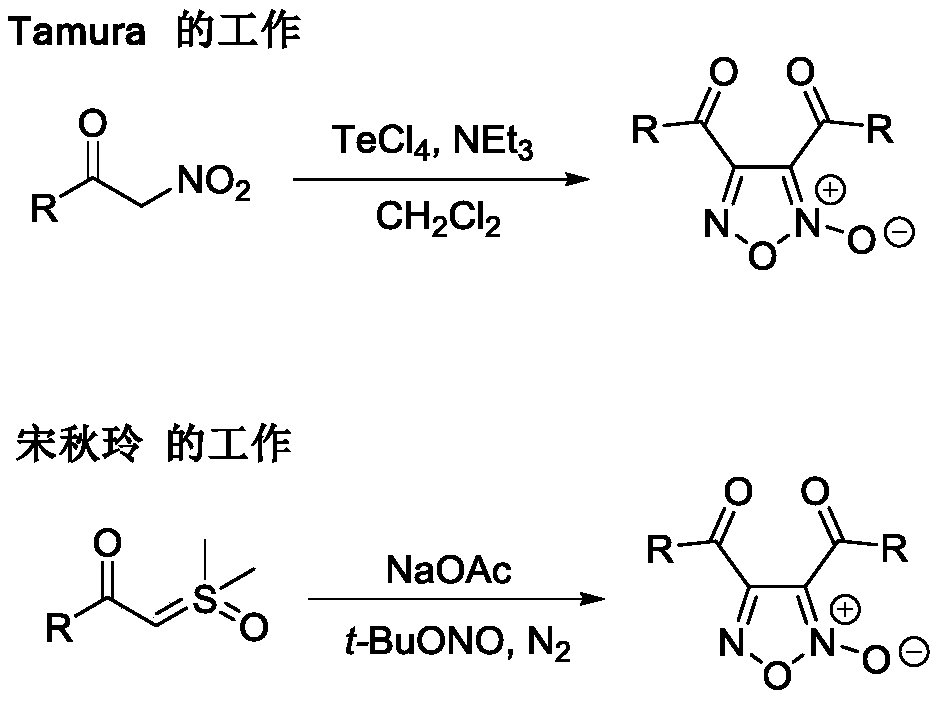
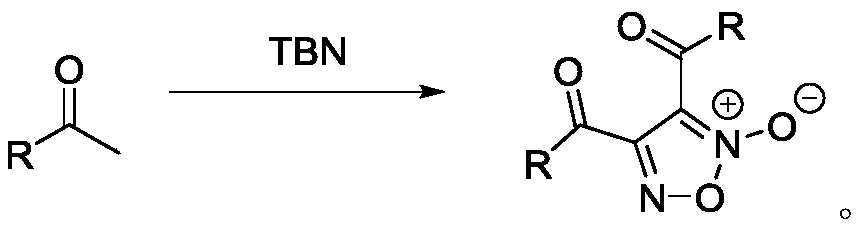
Method for directly synthesizing furoxan from methyl ketone
A technology of furoxan and methyl ketone, which is applied in the direction of organic chemistry, can solve the problems that raw materials cannot be purchased through commercial channels, limit the application prospects of synthetic methods, and reduce atom economy, so as to reduce the difficulty of post-processing of products and improve the quality of products. Universal applicability and effect of saving workload
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
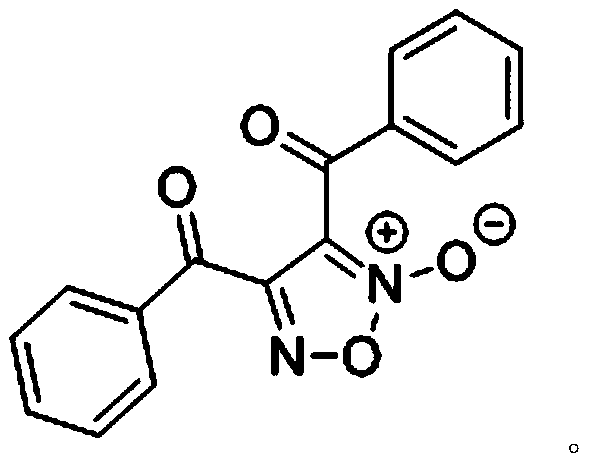
[0019] Specific embodiment 1: methyl ketone is acetophenone
[0020] Add 1 mmol of acetophenone into the reaction flask, and dissolve it in 1,4-dioxane (1 mL), add 528 μL of tert-butyl nitrite dropwise under stirring at room temperature, then raise the temperature to 60°C, and under air atmosphere, React for 24 hours. After the reaction, the organic solvent was removed with a rotary evaporator, purified by column chromatography or recrystallization to obtain 139 mg of furoxan product, and the reaction yield was 95%. Structure Identification: 1 H NMR (400MHz, Chloroform-d) δ8.20 (dd, J = 8.4, 1.2Hz, 2H), 7.86 (dd, J = 8.4, 1.2Hz, 2H), 7.70 (dt, J = 13.7, 7.5Hz, 2H), 7.54 (dt, J=13.8, 7.9Hz, 4H). 13 C NMR (100MHz, Chloroform-d) δ181.8, 180.5, 154.3, 135.5, 135.3, 133.87, 133.85, 130.6, 129.7, 129.3, 129.0, 111.6. HRMS (ESI): calcd for C 16 h 10 N 2 o 4 Na(M+Na) + :317.0533.Found:317.0529. The structural formula of furoxan is:
[0021]
specific Embodiment approach 2
[0022] Specific embodiment 2: methyl ketone is p-methyl acetophenone
[0023] Add 1mmol of p-methylacetophenone into the reaction flask and dissolve it in 1,4-dioxane (1mL). With stirring at room temperature, add 528μL of tert-butyl nitrite dropwise, then raise the temperature to 60°C and air Under atmosphere, react for 24 hours. After the reaction, the organic solvent was removed with a rotary evaporator, purified by column chromatography or recrystallization to obtain 155.5 mg of furoxan product, and the reaction yield was 97%. Structure Identification: 1 H NMR (400MHz, Chloroform-d) δ8.10(d, J=7.8Hz, 2H), 7.75(d, J=7.8Hz, 2H), 7.34(d, J=8.0Hz, 2H), 7.31(d ,J=8.1Hz,2H),2.46(s,3H),2.44(s,3H). 13 CNMR (100MHz, Chloroform-d) δ181.3, 180.0, 154.6, 147.0, 146.8, 131.49, 131.46, 130.7, 130.0, 129.84, 129.76, 111.9, 22.01, 21.97. HRMS (ESI): calcd for C 18 h 14 N 2 o 4 Na(M+Na) + :345.0846.Found:345.0839. The structural formula of furoxan is:
[0024]
specific Embodiment approach 3
[0025] Specific embodiment 3: methyl ketone is p-phenylacetophenone
[0026] Add 1 mmol of p-phenylacetophenone into the reaction flask, and dissolve it in 1,4-dioxane (1 mL), add 528 μL of tert-butyl nitrite dropwise under stirring at room temperature, then raise the temperature to 60 °C, and air Under atmosphere, react for 24 hours. After the reaction, the organic solvent was removed with a rotary evaporator, purified by column chromatography or recrystallization to obtain 215 mg of furoxan product, and the reaction yield was 96%. Structure Identification: 1 H NMR (400MHz, Chloroform-d) δ8.31 (d, J = 8.6Hz, 2H), 7.96 (d, J = 8.4Hz, 2H), 7.77 (dd, J = 14.4, 8.4Hz, 4H), 7.69 –7.60(m,4H),7.55–7.40(m,6H). 13 C NMR (100MHz, Chloroform-d)δ181.3,180.0,154.6,148.3,148.1,139.3,132.6,132.5,131.3,130.4,129.14,129.11,128.8,127.9,127.7,127.44,127.43.I11) calcd for C 28 h 18 N 2 o 4 H(M+H) + :447.1339.Found:447.1348. The structural formula of furoxan is:
[0027]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com