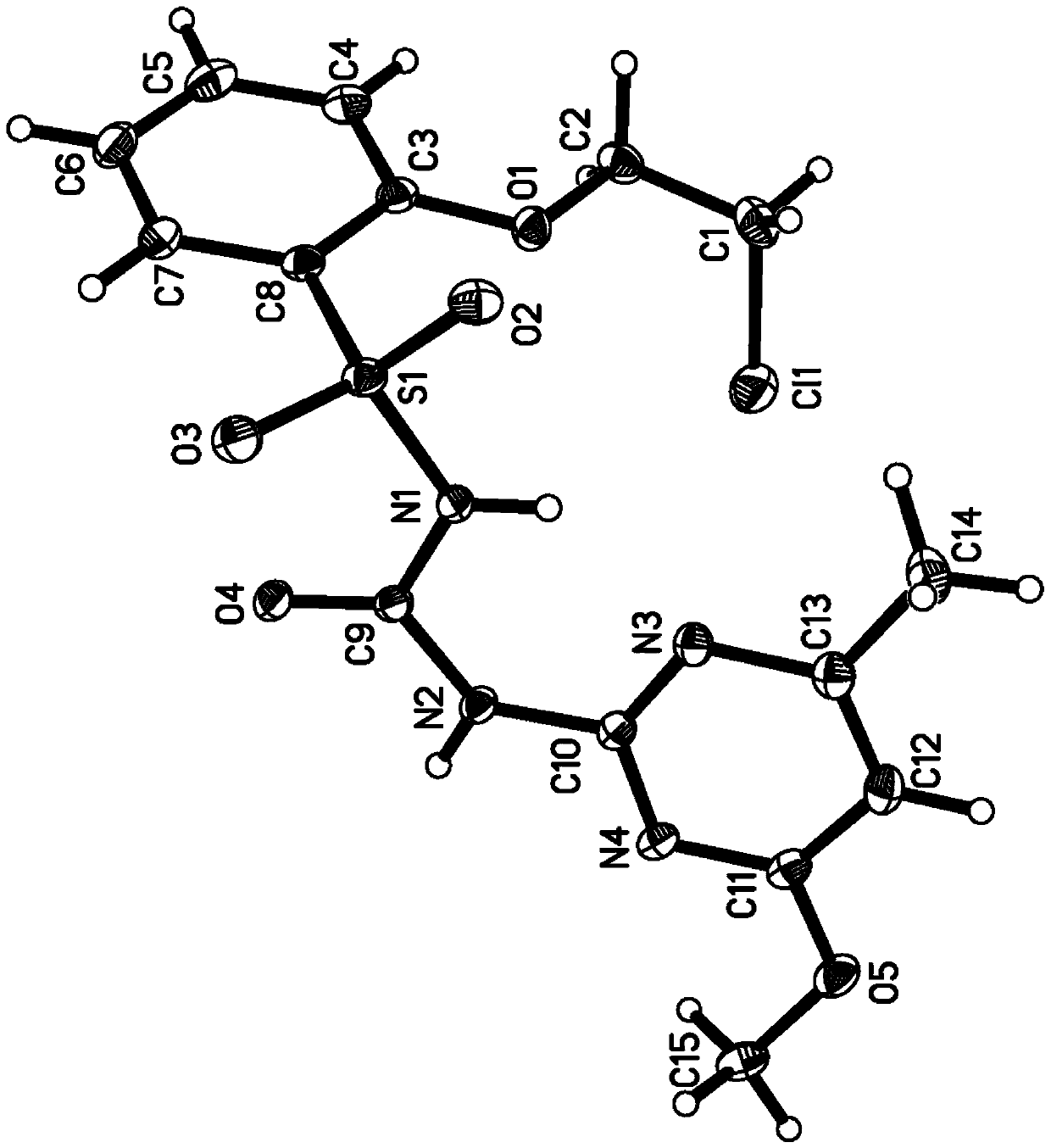
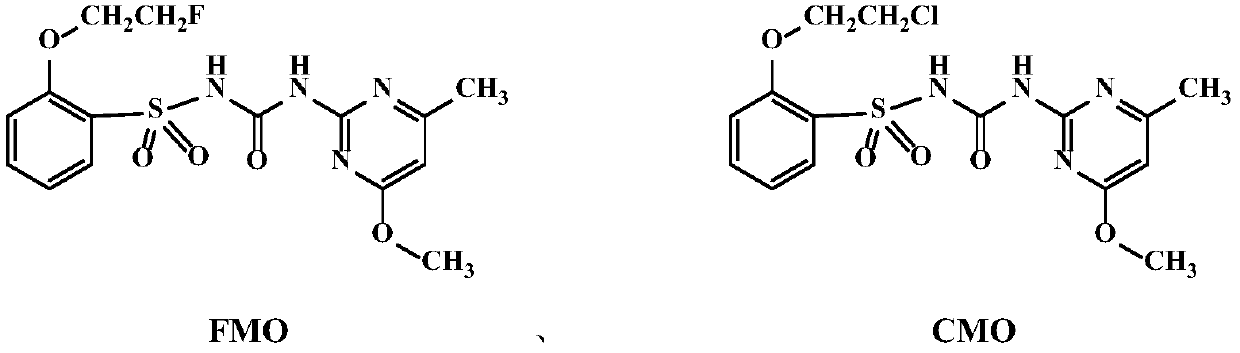
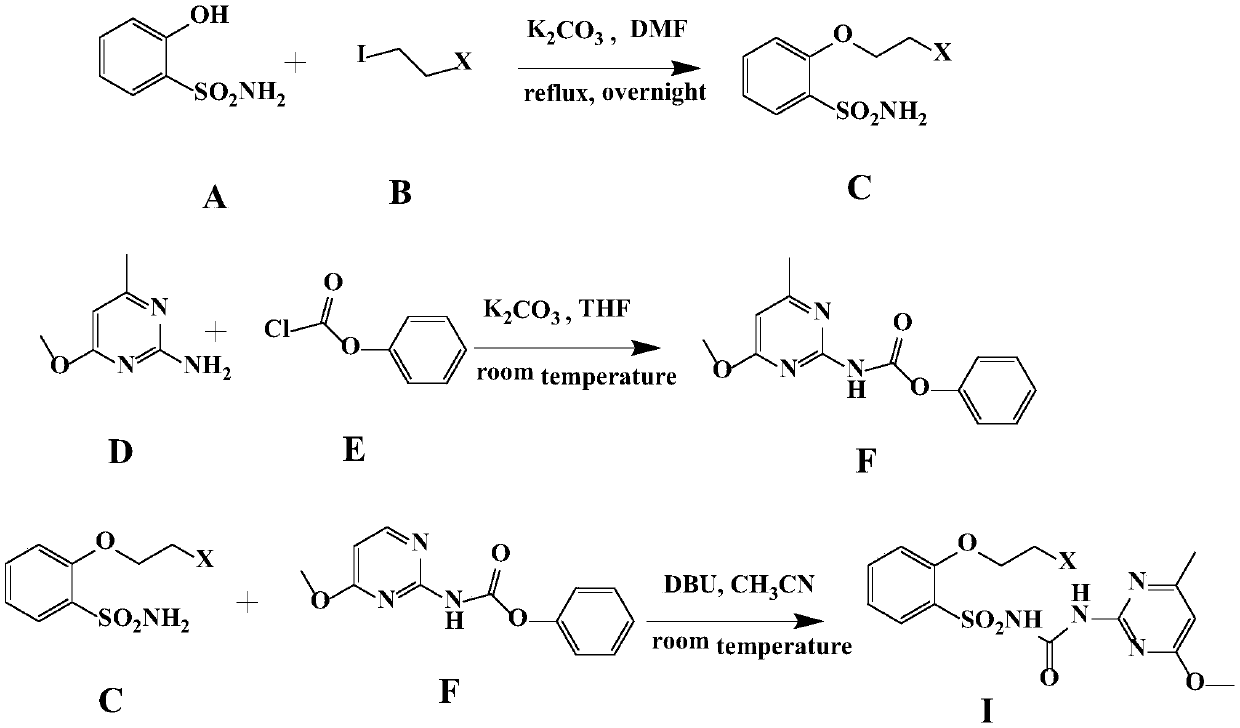
Sulfonylurea compound, preparation method thereof and use in preparing herbicide
A technology of compound and sulfonylurea, which is applied in the field of preparation of herbicides for the control of barnyardgrass, a malignant weed, can solve the problems of unselected safety and poor control effect of crops
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1. Preparation of compound FMO
[0022] Dissolve 8.65g (50mmol) of o-hydroxybenzenesulfonamide in 150mL of N,N-dimethylformamide, then add 34.5g (250mmol) of potassium carbonate, and stir the system at room temperature for 30 minutes. Then, 4.1 mL (50 mmol) of 1-fluoro-2-iodoethane was added dropwise to the reaction liquid, the temperature was raised to 100° C., and the reaction was refluxed overnight. Then filter and take the filtrate, add 300mL water and ethyl acetate (100mL×3) for extraction, dry the organic phase, and separate the product by column chromatography to obtain white to light yellow solid 2-(2-fluoroethoxy)benzenesulfonamide 3.7 g, yield 34%. of the intermediate 1 H NMR data is: (400MHz, CDCl 3 )δ7.94(dd, J=7.8,1.3Hz,1H,ArH),7.55(td,J=8.1,1.2Hz,1H,ArH),7.13(t,J=7.6Hz,1H,ArH),7.03 (d,J=8.3Hz,1H,ArH),5.11(s,2H,NH),4.89(ddd,J=47.2,4.9,3.0Hz,2H,CH 2 CH 2 F), 4.49–4.32 (m, 2H, CH 2 CH 2 F).
[0023] 7.0g (50mmol) of 2-amino-4-methoxyl-6-me...
Embodiment 2
[0029] Embodiment 2, compound are to the herbicidal effect of four kinds of model weeds on the pot test model
[0030] Liquid preparation
[0031] Preparation of emulsified water: first prepare a water emulsion with a content of 1‰, weigh 1g emulsifier with a beaker, add a small amount of distilled water to fully dissolve it, put it into a 1000mL volumetric flask, wash the beaker with distilled water several times, and pour it all into the volumetric flask , and finally add to the mark by distillation, shake well and set aside.
[0032] Mother liquor preparation: Weigh 30 mg of the test sample, add 1 mL of DMSO to fully dissolve it, and prepare a 30 mg / mL mother liquor. After calculating the dosage according to the spray area, pipette the required volume into a 10mL small beaker, and add the corresponding volume of emulsified water to make an aqueous emulsion for spraying. If necessary, serially dilute to obtain the desired aqueous emulsion for later use.
[0033] Spray equ...
Embodiment 3
[0040] Embodiment 3, the broad-spectrum herbicidal effect of compound to various weeds
[0041] The specific experimental details of the soil treatment method and the stem and leaf treatment method are the same as in Example 2. The compounds tested in the experiment are FMO, CMO, and the commercial herbicide trisulfuron-methyl, and the weed types are thrush, barnyardgrass, and amansia Wheat, goosegrass, foxtail, soda, echinacea, chrysanthemum, bittern, purslane, morning glory, velvetleaf, alfalfa, shepherd's, ashweed, dandelion, polygonum, and barnyardgrass at a dose of 1 g / mu. Table 3 shows the broad-spectrum herbicidal effects of the compounds.
[0042] Table 3, the broad-spectrum control effect of test compound on various weeds (1 gram / mu dose, percent inhibition rate)
[0043]
[0044]
[0045] * - Indicates that the herbicidal results under this condition were not measured due to insufficient crop seeds tested.
[0046] It can be seen from the table that FMO and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com