Applications of high-charge cationic porphyrin in preparation of PDT nanometer photosensitizer
A photosensitizer and cation technology, which is applied in the application field of highly charged cationic porphyrin in the preparation of PDT nano photosensitizers, can solve the problems of reducing the potential treatment of deep tumors, low tissue penetration ability, and short absorption wavelength, and achieves high photoresistance. Cytotoxicity, good EPR effect, effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A kind of preparation of PDT nano photosensitizer:
[0039] (1) Fmoc-H / Zn 2+ Preparation of:
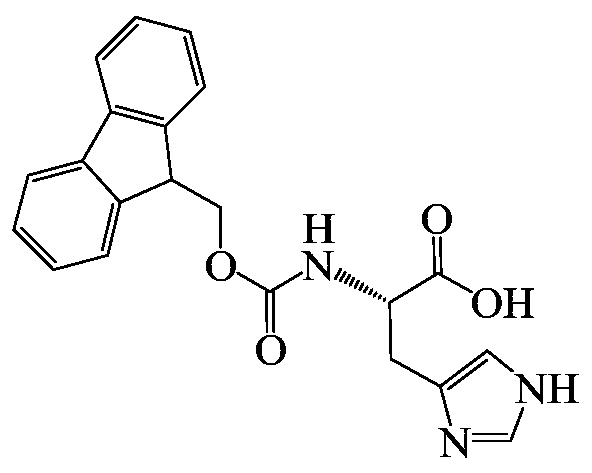
[0040] Fluorenylmethoxycarbonyl-L-histidine (hereinafter referred to as Fmoc-H) was dissolved in 50mM HCl to prepare a Fmoc-H solution with a concentration of 10mg / mL; then, the Fmoc-H solution and ZnCl 2 The solution was added to water to prepare a final concentration of 0.5 mM ZnCl 2 and an aqueous mixture of Fmoc-H at 1 mg / mL; the pH of the mixture was adjusted to neutral by adding 1M Tris solution, and self-assembled to form nanospherical particles Fmoc-H / Zn 2+ .
[0041] (2) Fmoc-H / Zn 2+ Preparation of / OMHEPzEOPP:
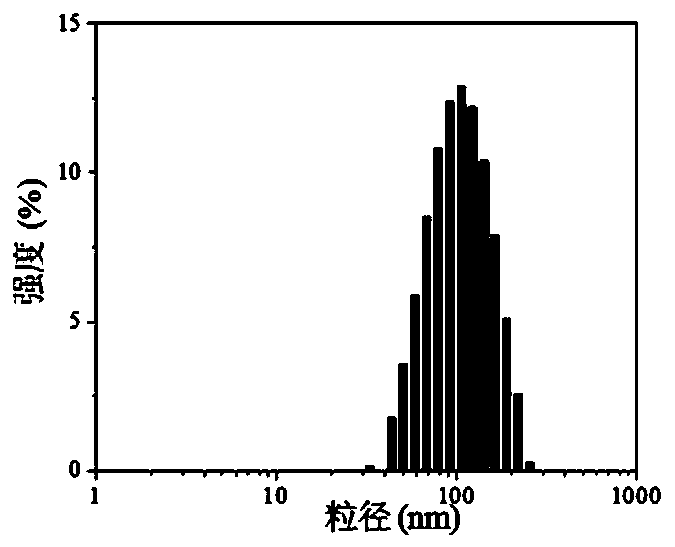
[0042] Dissolve the porphyrin OMHEPzEOPP in a Tris solution with a concentration of 50mg / mL 2M as a stock solution of porphyrin; to the Fmoc-H / Zn prepared above 2+ Add OMHEPzEOPP dissolved in 2M Tris-HCl buffer to the final concentration of OMHEPzEOPP to 1mg / mL to obtain a mixture; mix this mixture and place it in a centrifuge for centrifugation, remove...
Embodiment 2
[0047] (1) Cellular uptake and confocal microscopy imaging.
[0048] (A) Cell culture. The incubator was maintained at 37 °C, 5% CO 2 , HeLa cells were cultured in DMEM containing 10% fetal bovine serum and 1% double antibody.
[0049] (B) Adding nanocomposite photosensitizer. Fmoc-H / Zn 2+ / OMHEPzEOPP / AS1411 (the concentration of OMHEPzEOPP is 0.5μM) was co-incubated with Hela cells for 1h, 4h, 12h, and 24h, respectively recorded as samples 1, 2, 3, and 4, and washed 3 times with PBS to remove uningested drugs , and then fix the cells with 4% paraformaldehyde for 15 min.
[0050] (C) Experimental results obtained using laser confocal imaging. Olympus IX-81 microscope was used for laser confocal imaging, and 458nm was selected as the excitation wavelength of the diode-pumped laser. Obtain nanocomposite photosensitizer Fmoc-H / Zn 2+ Confocal fluorescence microscopy images of / OMHEPzEOPP / AS1411 co-incubated with HeLa cells for 1h, 4h, 12h, 24h. (See Figure 4a to Figure 4...
Embodiment 3
[0061] Preparation of a highly charged cationic porphyrin:
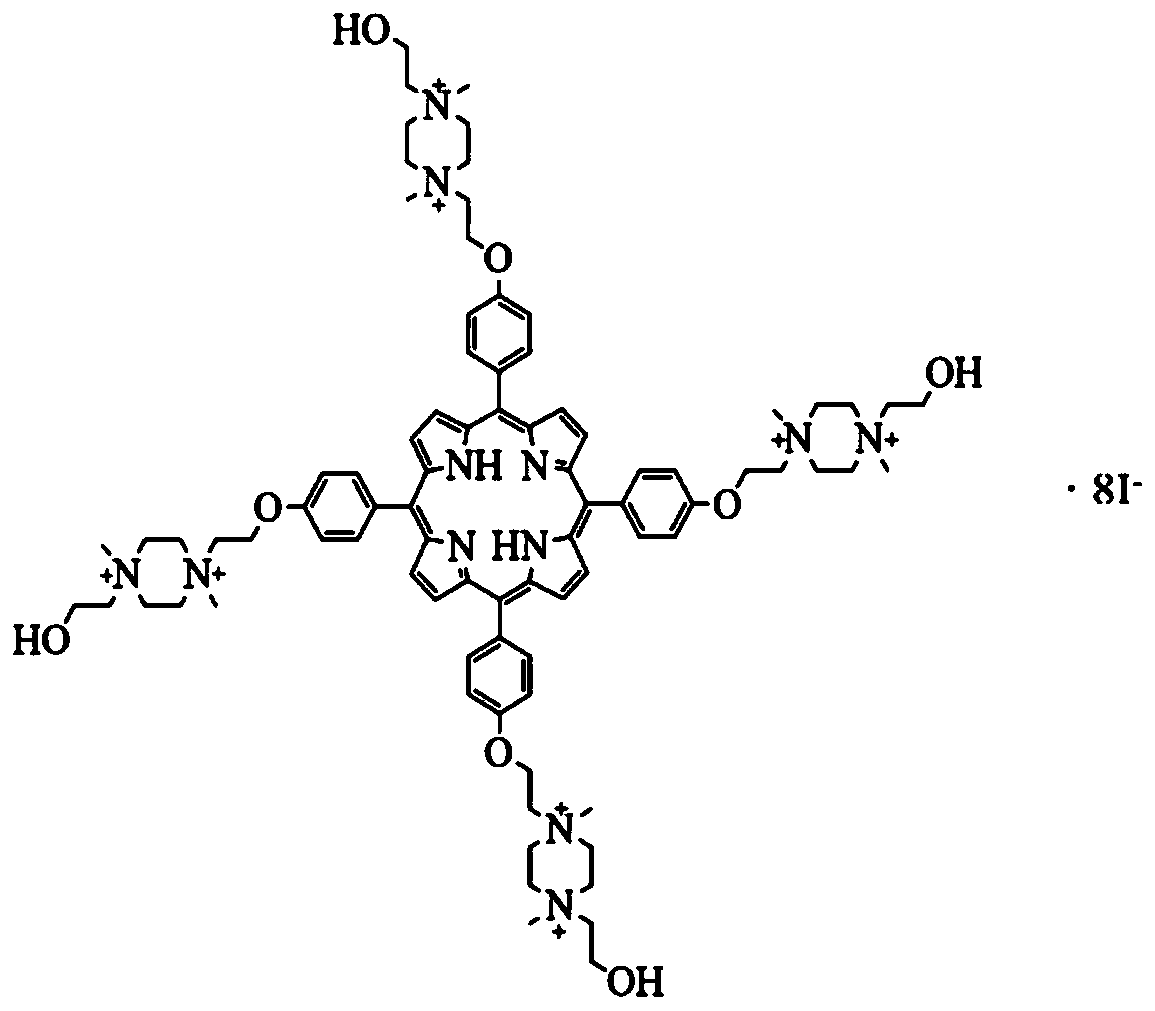
[0062] Highly charged cationic porphyrin chemical name: octaiodide 5,10,15,20-tetra{4-[2-[1,4-dimethyl-4-(2-hydroxyethyl)piperazine-1- base] ethoxy] phenyl} porphyrin. (OMHEPzEOPP for short), see figure 1 .
[0063] (1) Synthesis of 5,10,15,20-tetrakis[4-(2-bromoethoxy)phenyl]porphyrin
[0064] Add 5,10,15,20-tetrakis(4-hydroxyphenyl)porphyrin 0.23mM, 36mM K 2 CO 3 , 40mL N,N-dimethylformamide, magnetic stirring at room temperature. Then, 23mM 1,2-dibromoethane was diluted with 10mL DMF under the condition of avoiding light, and added dropwise into the three-necked flask through a constant pressure separating funnel, and stirred evenly by magnetic force at room temperature. After the dropwise addition was completed, the reaction temperature was adjusted to 60° C. and stirred for 8 h. After the reaction was completed, the temperature was lowered to room temperature, filtered under reduced pressure, and potassiu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com