T7 bacteriophage tail fibrin polypeptide and application thereof
A fibrin and bacteriophage technology, applied in the field of book genetic engineering, can solve the problems of low yield and not always improving the solubility of scFv
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
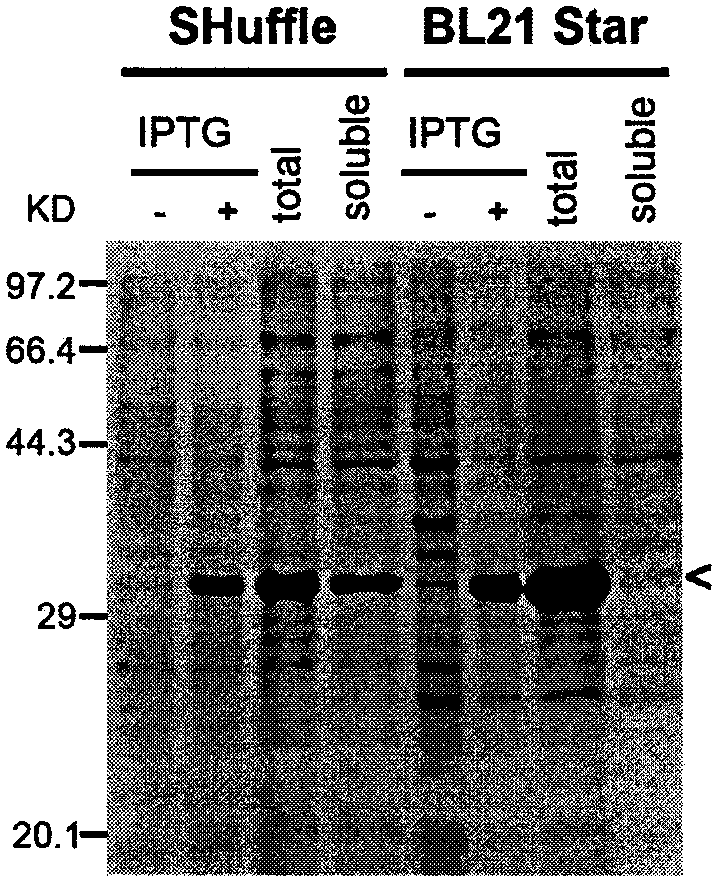
[0035] Example 1: Soluble expression and purification of G12-scFv in SHuffle T7 strain
[0036] The pET28a-His-G12-scFv-HA recombinant plasmid expressing G12-scFv was transformed into Escherichia coli BL21(DE3)star and SHuffle strains respectively; under the same culture conditions, protein expression was induced with 0.5mM IPTG at 30°C. According to the weight of the bacteria, add the bacterial lysate in proportion, then crush the bacteria by ultrasonic, and divide them into soluble and insoluble components by centrifugation; the total bacterial protein obtained by SDS cracking before / after IPTG induction, the total bacterial protein obtained by ultrasonic crushing and the soluble Proteins were first separated by SDS-PAGE and then analyzed by Coomassie brilliant blue staining. In the absence of ITPG induction, SHuffle and BL21Star expressing bacteria hardly expressed G12-scFv ( figure 1 , IPTG-); After induction with 0.5mM ITPG, the expression of G12-scFv in the two strains...
Embodiment 2
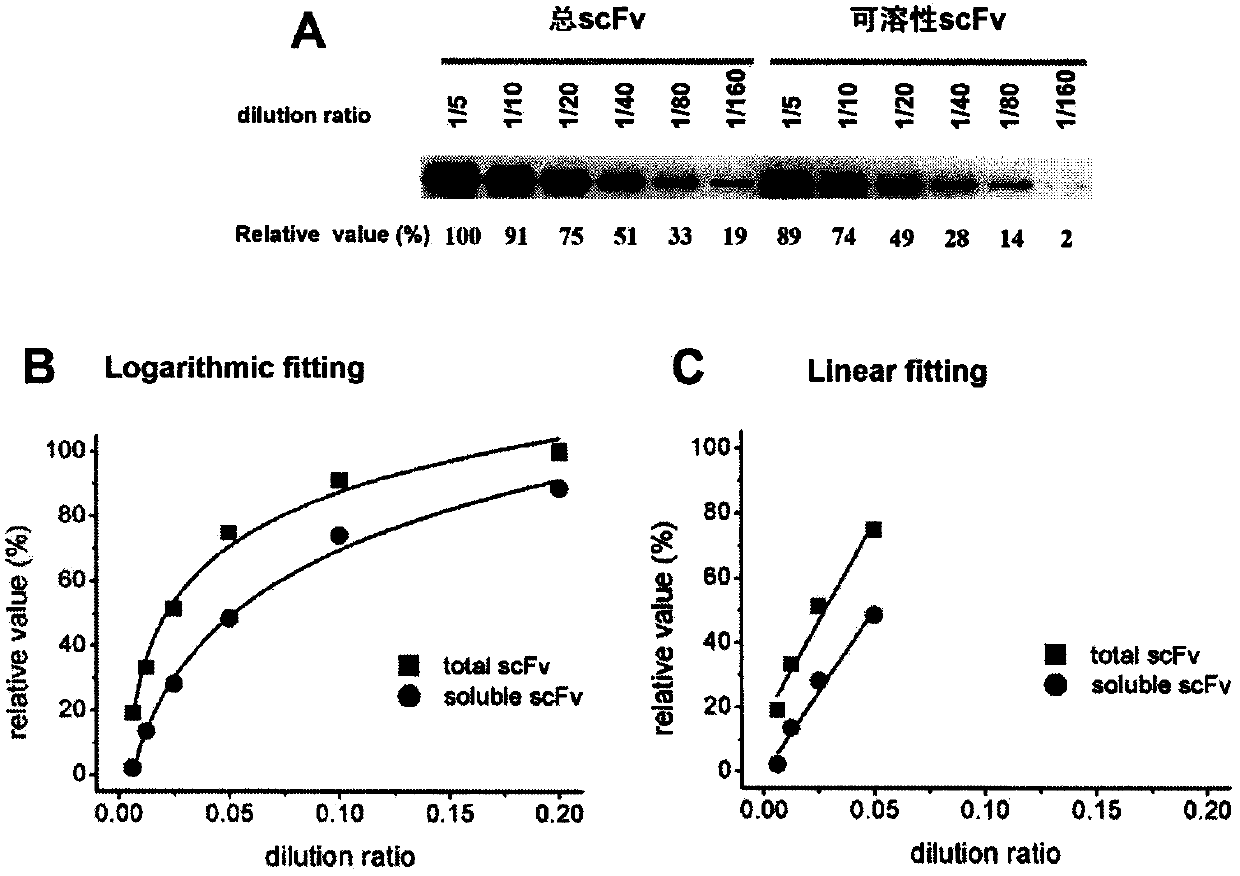
[0037] Embodiment 2: Doubling dilution and Western blotting determine the linear dilution range of bacterial protein sample
[0038] Among the present invention, the solubility of scFv is represented by the percentage value of soluble protein / total protein; first obtain bacterial total protein and soluble protein according to the method of embodiment 1, then do multiple dilution to these two samples, then by known Westernblotting technique Detection of G12-scFv-P17 total protein and soluble protein ( figure 2 A), at last by MultiGauge software, do gray-scale scanning quantification and determine the linear dilution range ( figure 2 B and 2C), such as figure 2 As shown in B and 2C, when the dilution ratio of bacterial total protein and soluble protein is in the interval of 1 / 20-1 / 160, the gray-scale scanning value is linearly correlated with the dilution ratio. The dilution ratio is controlled between 1 / 20-1 / 160.
Embodiment 3
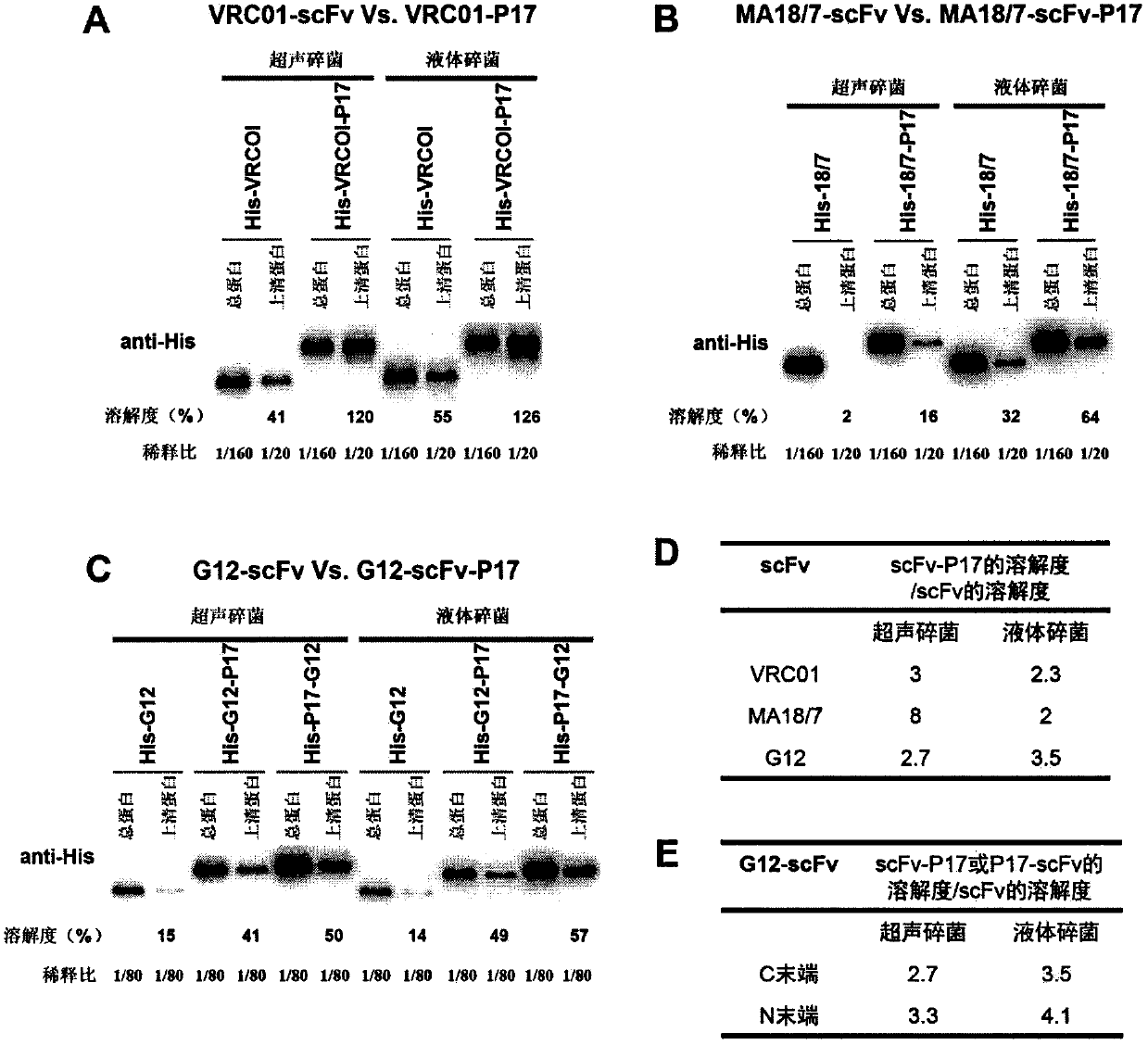
[0040] P17 can increase the solubility of three scFv fusion proteins in E. coli SHuffle strain by 2-8 times
[0041] The recombinant expression vectors of G12, MA18 / 7 and VRC01 were transformed into the SHuffle strain, and then the bacterial total protein and soluble protein were prepared by ultrasonic and liquid crushing methods according to the method described in Example 1. 1 / 20-1 / 160 linear interval dilution, then Western blotting detection with His tag antibody and grayscale scanning quantification with MultiGauge software, and finally calculate and compare the solubility, P17 located at the carboxyl terminal of scFv can increase VRC01-scFv ( image 3 A), MA18 / 7-scFv ( image 3 B) and G12-scFv ( image 3 C) Solubility in SHuffle strains, and the solubilizing effect is not dependent on the bacterial lysis mode, and the solubilizing effect is 2-8 times ( image 3 D).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com