Pharmaceutical composition for preventing and treating cerebral infarction
A composition and technology of cerebral infarction, applied in the field of medicine, can solve the problems of slow onset of effect and general therapeutic effect, and achieve the effect of no side effects, good therapeutic effect and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
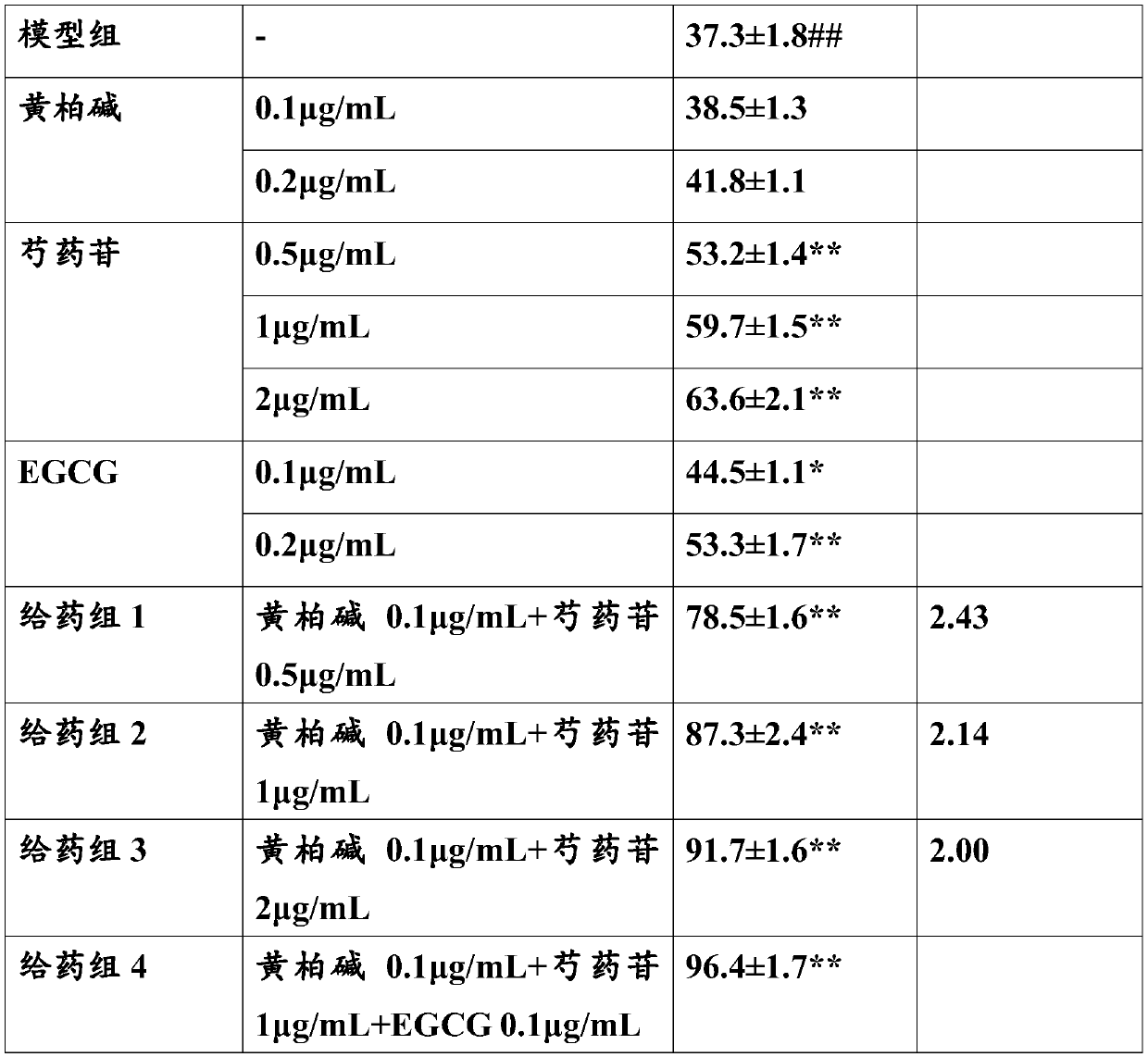
[0032] Example 1 The protective effect of the pharmaceutical composition on the model of hypoxia and glucose deficiency of nerve cells
[0033] Cell culture: Take the frozen PC12 cell line and resuscitate it in a centrifuge tube, centrifuge to get the supernatant, suspend it with RPMI1640 medium containing 10% FBS, inoculate it in a petri dish, culture and pass in an incubator, and grow in logarithm Harvest the cells at period, adjust the concentration to 1×10 by suspending in medium 4 cells / ml were plated in 24-well plates for subsequent experiments.
[0034] Experimental grouping: normal control group, continue to pass through 95% O 2 and 5% CO 2 The mixed gas; the second group is the OGD model group: PC12 cells remove the normal culture medium and replace it with glucose-free HBSS solution (140mM NaCl, 3.5mMKCl, 12mM MgSO 4, 5mM NaHCO 3 ,1.7mM CaCl 2 ,0.4mM KH 2 PO 4 , 10mM Hepes), put into a dedicated anoxic tank, and pass through 95% N 2 and 5% CO 2 In the treat...
Embodiment 2
[0048] Embodiment 2: The influence of medicine on rat cerebral ischemia-reperfusion model
[0049] experiment material
[0050] Animals: healthy Wistar rats, domesticated in the animal breeding room for more than 3 days, all male, weighing 180-220 g, a total of 80 rats.
[0051] experiment method
[0052] Wistar rats were anesthetized with isoflurane (3% for introduction, 2% for maintenance) using anesthesia to dissect the right carotid artery by median incision, and after the common carotid and external carotid arteries were threaded, they were temporarily blocked Blood flow in the common and external carotid arteries. An embolus was inserted from the external carotid artery, and after cutting the external carotid artery, it was inserted 10.5 mm into the internal carotid artery from the bifurcation of the external carotid artery and the internal carotid artery, and the start of the middle cerebral artery was closed to close the surgical wound. The rectal temperature of the...
Embodiment 3
[0067] Embodiment 3: pharmaceutical composition tablet of the present invention
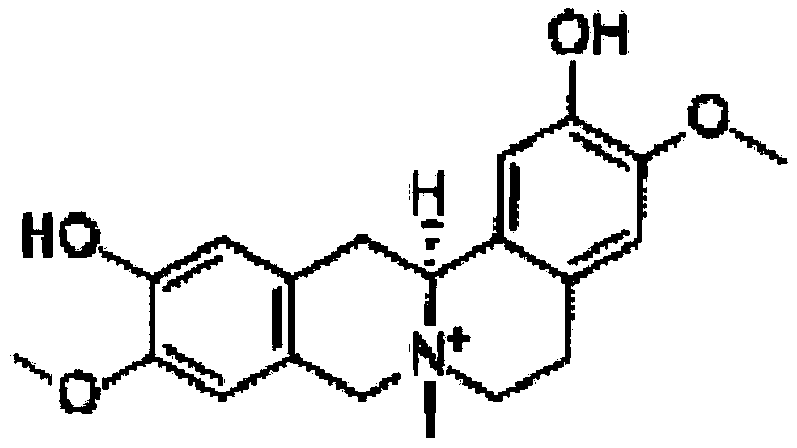
[0068] Weigh 8g of cortex base, 16g of paeoniflorin, mix well, add 160g of sodium hydroxymethyl cellulose, 50g of dextrin, pass through a 16-mesh sieve to make wet granules, dry the wet granules at 50-60°C, and dry the granules with a 20-mesh Sieve for granulation, add 2% magnesium stearate, mix well, and press into tablets with a weight of 0.2g / tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com