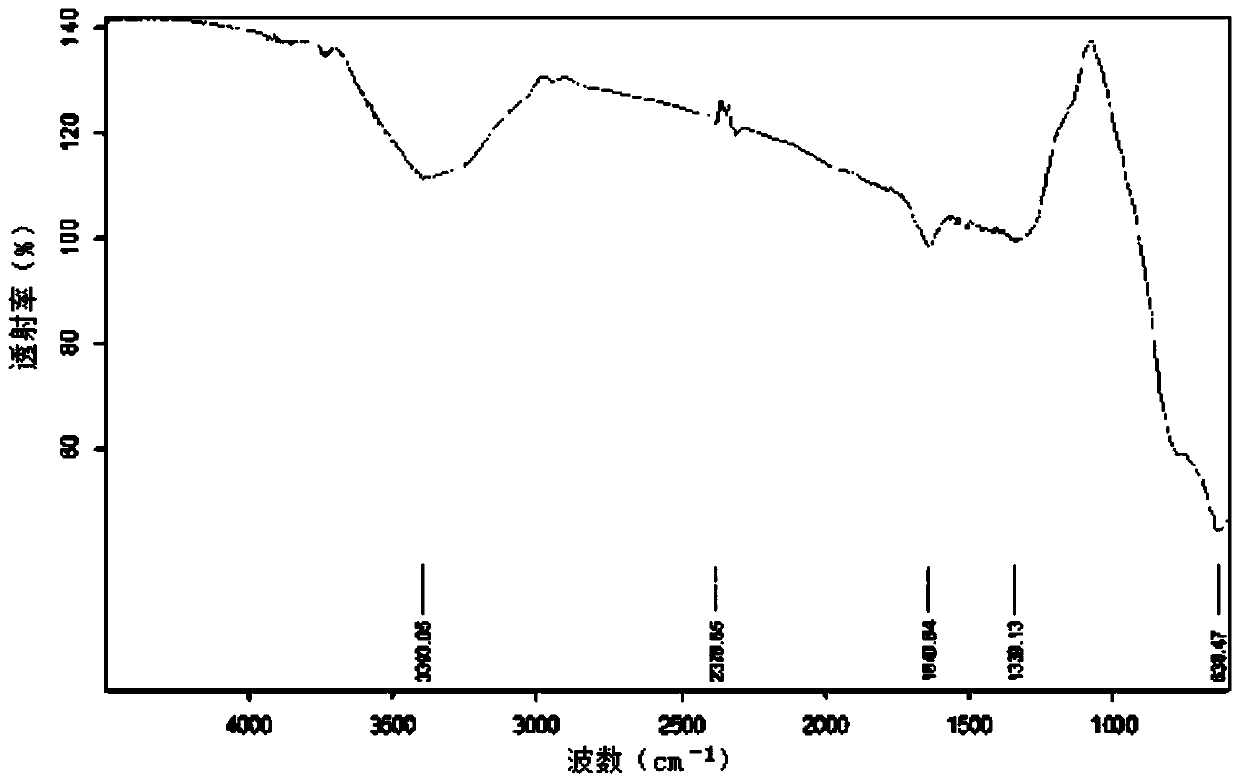
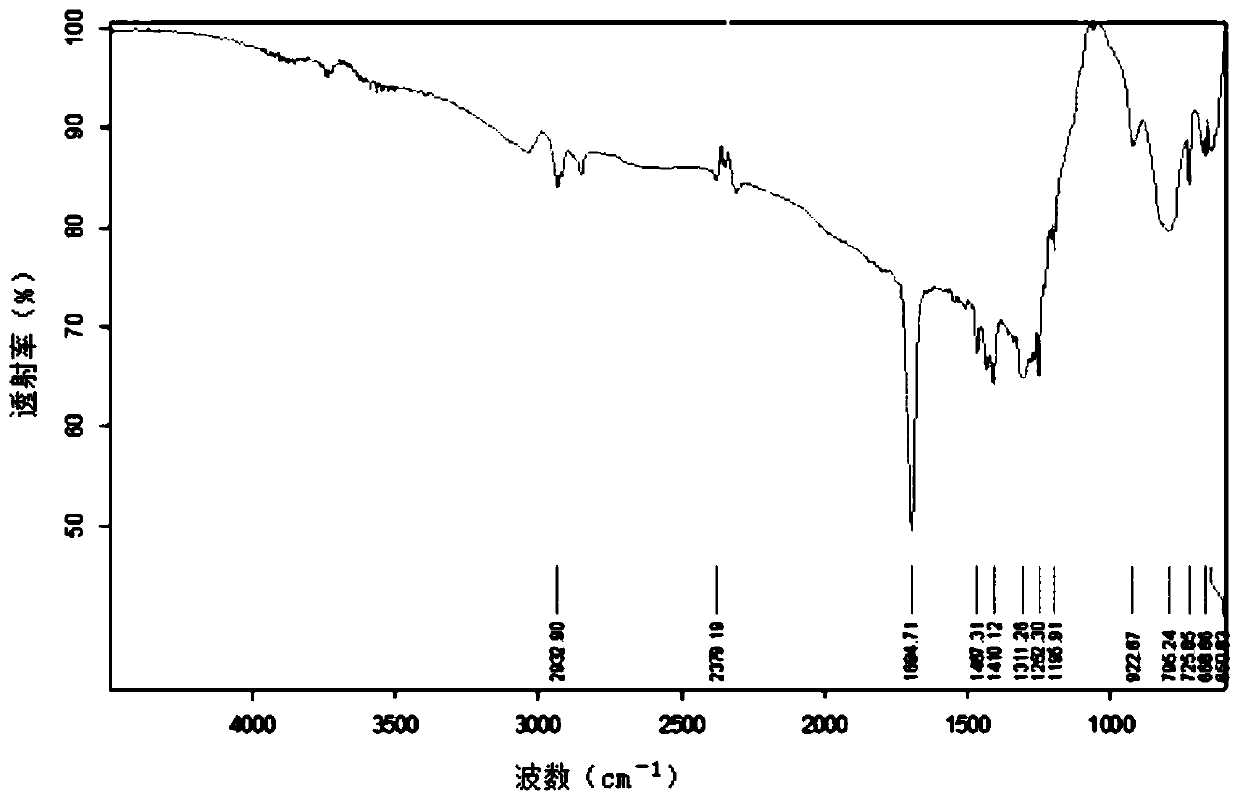
Preparation method of azelaic acid supramolecules
A kind of azelaic acid and supramolecular technology, applied in the field of supramolecular chemistry, can solve the problem of less azelaic acid and the like, and achieve the effects of treating acne, improving compatibility and increasing stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] A supermolecule of azelaic acid, molecular recognition is carried out by azelaic acid, polyethylene glycol-400, and propylene glycol, calculated according to the mass ratio, wherein the ratio of azelaic acid: polyethylene glycol-400: propylene glycol is 0.4:15:5.25.
[0030] The preparation method of described azelaic acid supramolecular comprises:
[0031] (1) Measure 11.8mL of polyethylene glycol-400 (15g) and 5mL of 1,3-propanediol (5.25g) in a beaker, mix them, and stir at 600r / min for 8min to obtain a mixed solvent.
[0032] (2) Weigh 0.4 g of azelaic acid and dissolve it in the above solvent, and stir in a constant temperature water bath at 50° C. at 500 r / min for 15 min to form an azelaic acid solution.
[0033] (3) Measure 100 mL of deionized water into a beaker and heat to 98°C. Stir at a speed of 600r / min, and dropwise add the azelaic acid solution to the above-mentioned deionized water at a speed of 1mL / min. The dropping temperature of the azelaic acid solut...
Embodiment 2
[0036] A supermolecule of azelaic acid, molecular recognition is carried out by azelaic acid, polyethylene glycol-400, and propylene glycol, calculated according to the mass ratio, wherein the ratio of azelaic acid: polyethylene glycol-400: propylene glycol is 0.6:15:6.3.
[0037] (1) Measure 11.8mL of polyethylene glycol-400 (15g) and 6mL of 1,3-propanediol (6.3g) in a beaker, mix them, and stir at 600r / min for 8min to obtain a mixed solvent.
[0038] (2) Weigh 0.6 g of azelaic acid and dissolve it in the above solvent, and stir in a constant temperature water bath at 50° C. at 500 r / min for 15 min to form an azelaic acid solution.
[0039] (3) Measure 100 mL of deionized water into a beaker and heat to 98°C. Stir at a speed of 600r / min, and dropwise add the azelaic acid solution to the above-mentioned deionized water at a speed of 1mL / min. The dropping temperature of the azelaic acid solution is 50°C. Continue to stir at a constant speed for 2 hours to obtain supramolecular...
Embodiment 3
[0042] A supermolecule of azelaic acid, molecular recognition is carried out by azelaic acid, polyethylene glycol-400, and propylene glycol, calculated according to the mass ratio, wherein the ratio of azelaic acid: polyethylene glycol-400: propylene glycol is 0.8:15:7.35.
[0043] (1) Measure 11.8mL of polyethylene glycol-400 (15g) and 7mL of 1,3-propanediol (7.35g) in a beaker, mix them, and stir at 600r / min for 8min to obtain a mixed solvent.
[0044] (2) Weigh 0.8 g of azelaic acid and dissolve it in the above solvent, and stir in a constant temperature water bath at 50° C. at 500 r / min for 15 min to form an azelaic acid solution.
[0045] (3) Measure 100 mL of deionized water into a beaker and heat to 98°C. Stir at a speed of 600r / min, and dropwise add the azelaic acid solution to the above-mentioned deionized water at a speed of 1mL / min. The dropping temperature of the azelaic acid solution is 50°C. Continue to stir at a constant speed for 2 hours to obtain supramolecul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com