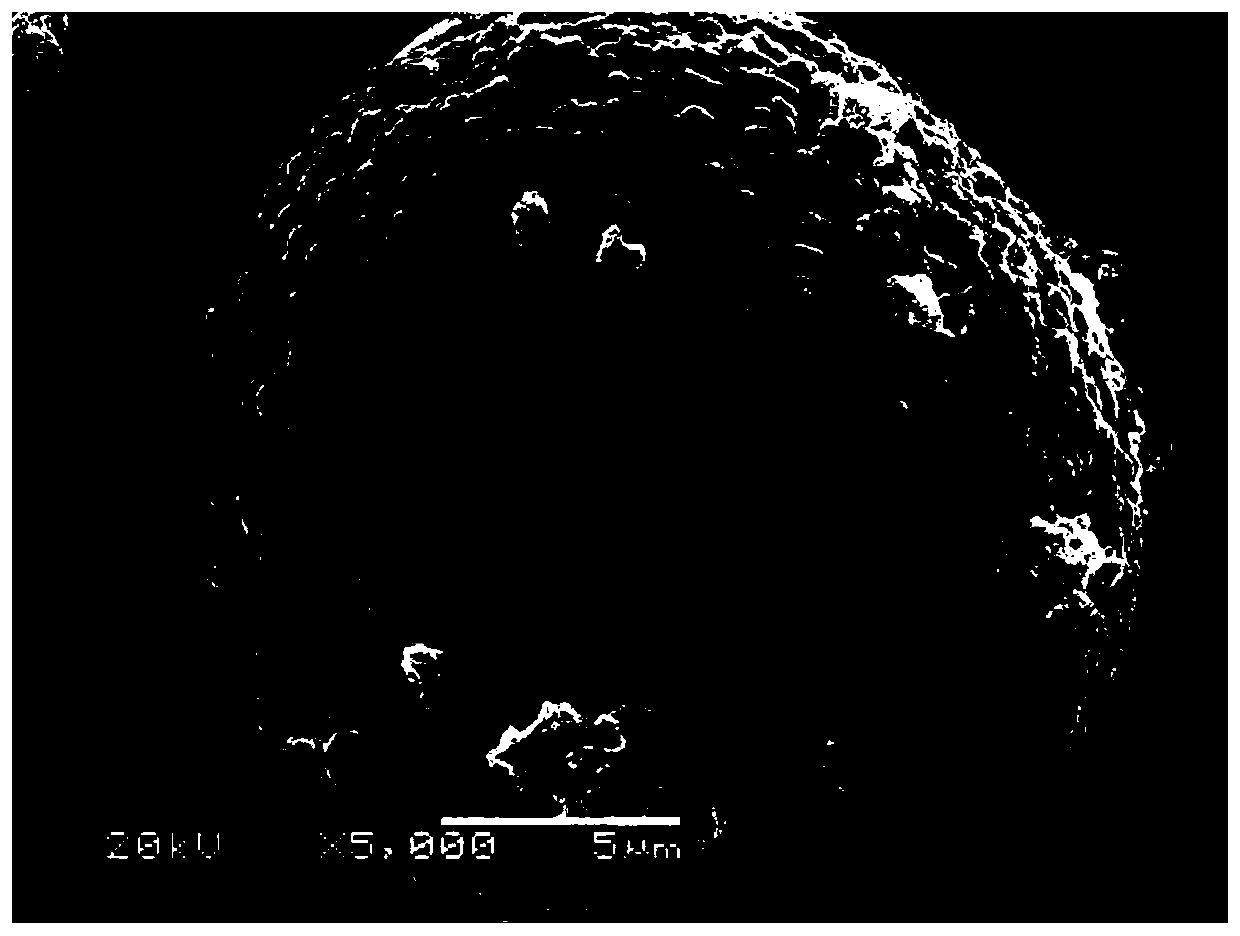
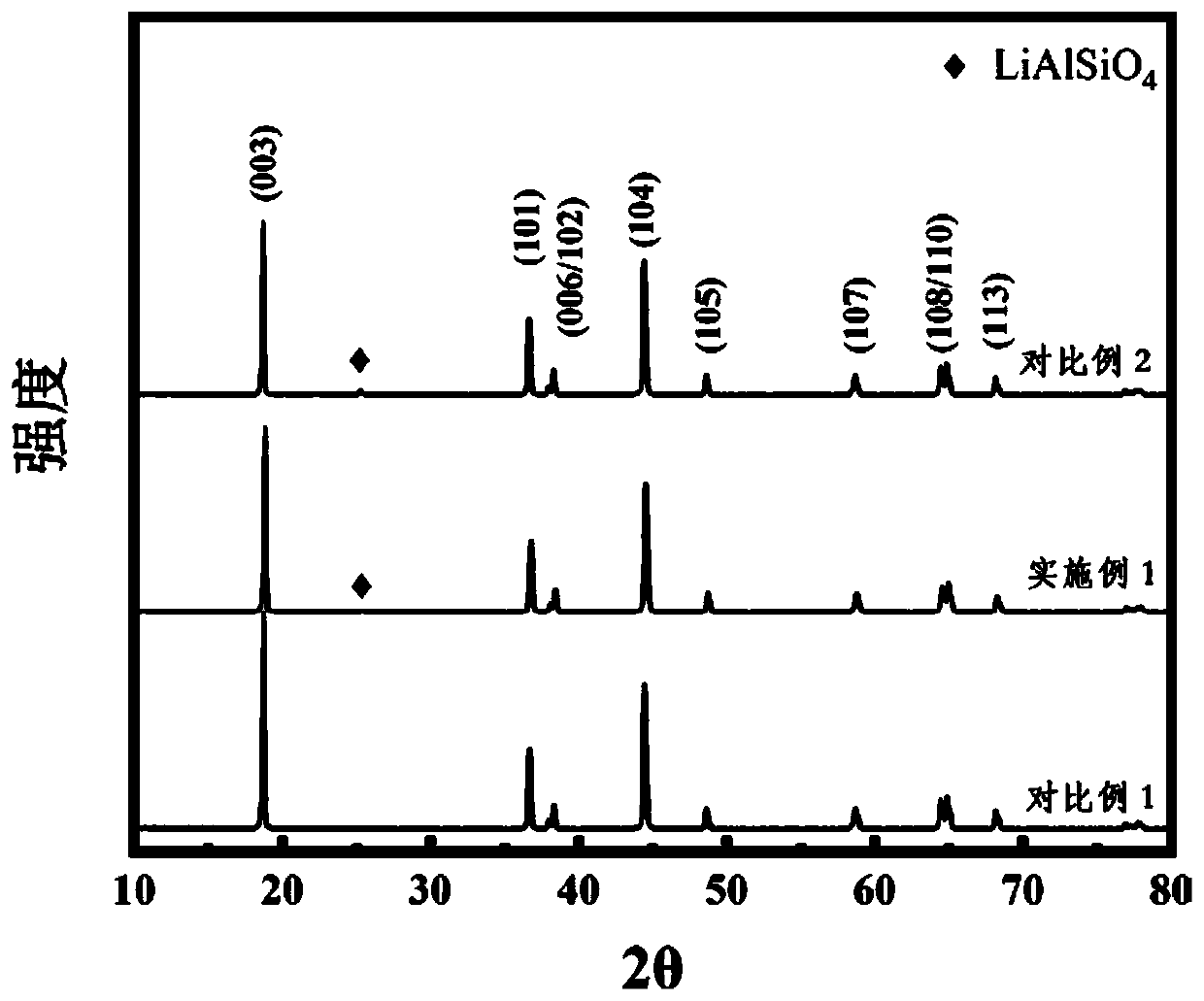
High-nickel ternary cathode material coated with fast ion conductor and preparation method thereof
A technology of ion conductor and positive electrode material, which is applied to the field of high nickel ternary positive electrode material coated with fast ion conductor and its preparation field, can solve the problems of increased internal resistance of battery and reduced discharge specific capacity, and achieves reduction of internal resistance of battery, The effect of reducing polarization and ensuring capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Weigh 0.1653g of ethyl orthosilicate C 8 h 20 o 4 Si(TEOS), 0.2977g of Al(NO 3 ) 3 9H 2 O and 0.0547 g of LiNO 3 Put it into a beaker, add 60mL of alcohol, and add 2.5g of citric acid as a templating agent, and keep stirring in an oil bath at 80°C for 3h.
[0037] (2) Weigh 10.0 g of the precursor, add it into the solution of (1), and keep stirring and mixing for 6 hours.
[0038] (3) When the solution in (2) is stirred at 100°C and becomes sol-gel, take it out and dry it in an oven at 120°C for 24 hours; grind the obtained solid to obtain a powder material of 1wt% lithium aluminum silicate-coated precursor ;
[0039] (4) After weighing a certain mass of the powder obtained in (3), subtract the mass of the coated substance, and add lithium hydroxide according to the molar ratio of lithium to transition metal element 1:1.05, (such as the mass of the precursor after deducting is 9.8 g, calculate the molar mass of the transition metal element in the precursor to ...
Embodiment 2
[0061] (1) Weigh 1.1571g of tetraethyl orthosilicate C 8 h 20 o 4 Si(TEOS), 2.0839g of Al(NO 3 ) 3 9H 2 O and 0.3829g of LiNO 3 Put it into a beaker, add 60mL of alcohol, and add 2.5g of citric acid as a templating agent, and keep stirring in an oil bath at 80°C for 3h.
[0062] (2) Weigh 10.0 g of the precursor, add it into the solution of (1), and keep stirring and mixing for 6 hours.
[0063] (3) After the solution in (2) is stirred into a sol-gel form, take it out and put it in an oven at 120° C. to dry for 24 hours; and grind the obtained solid evenly to obtain a 7wt% lithium aluminum silicate-coated precursor powder material;
[0064] (4) After weighing a certain mass of the powder obtained in (3), subtract the mass of the coated substance, add lithium hydroxide according to the molar ratio of lithium to transition metal element 1:1.05, and mix and mix;
[0065] (5) Pre-sinter the powder mixed in (4) at 480°C for 6 hours; sinter at 800°C for 15 hours, the sinterin...
Embodiment 3
[0073] (1) Weigh 0.1653g of ethyl orthosilicate C 8 h 20 o 4 Si(TEOS), 0.2977g of Al(NO 3 ) 3 9H 2 O and 0.0547 g of LiNO 3 Put it into a beaker, add 60mL of alcohol, and add 2.5g of citric acid as a templating agent, and keep stirring in an oil bath at 80°C for 3h.
[0074] (2) Weigh 10.0 g of the precursor, add it into the solution of (1), and keep stirring and mixing for 6 hours.
[0075] (3) After the solution in (2) is stirred into a sol-gel state, take it out and put it in an oven at 120° C. to dry for 24 hours; and grind the obtained solid evenly to obtain a powder material of 1 wt% lithium aluminum silicate-coated precursor;
[0076] (4) After weighing a certain mass of the powder obtained in (3), subtract the mass of the coating material, add lithium hydroxide according to the molar ratio of lithium to transition metal element 1:1.05, and mix it;
[0077] (5) Pre-sinter the powder mixed in (4) at 480°C for 6 hours; sinter at 900°C for 15 hours, the sintering he...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com