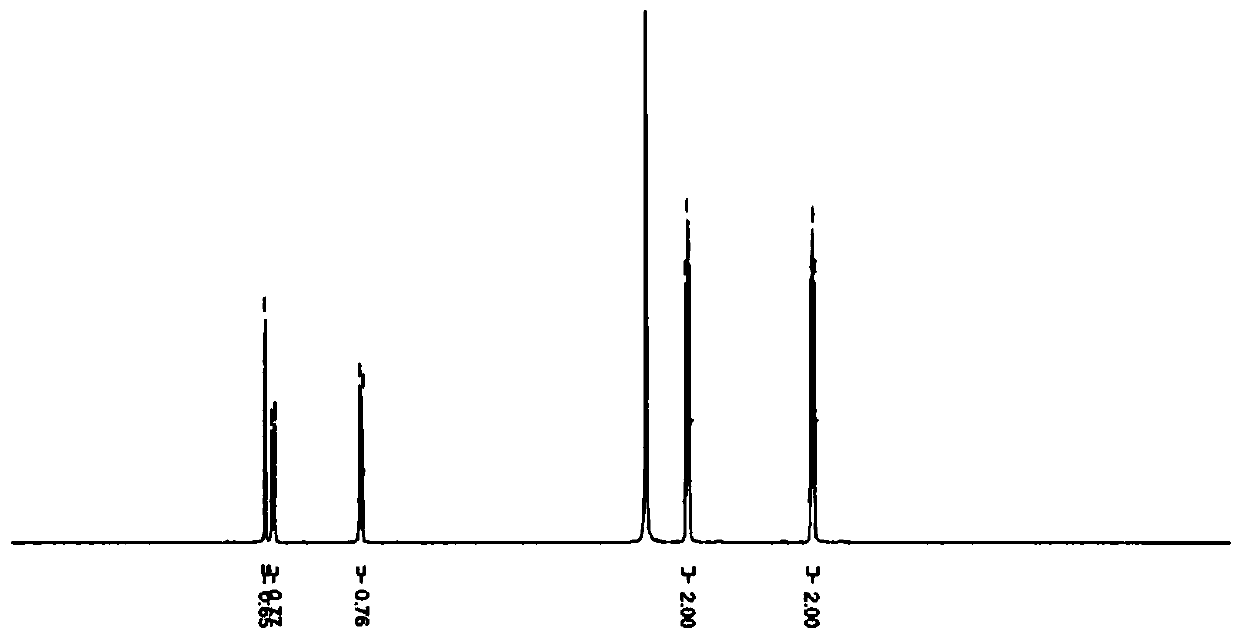
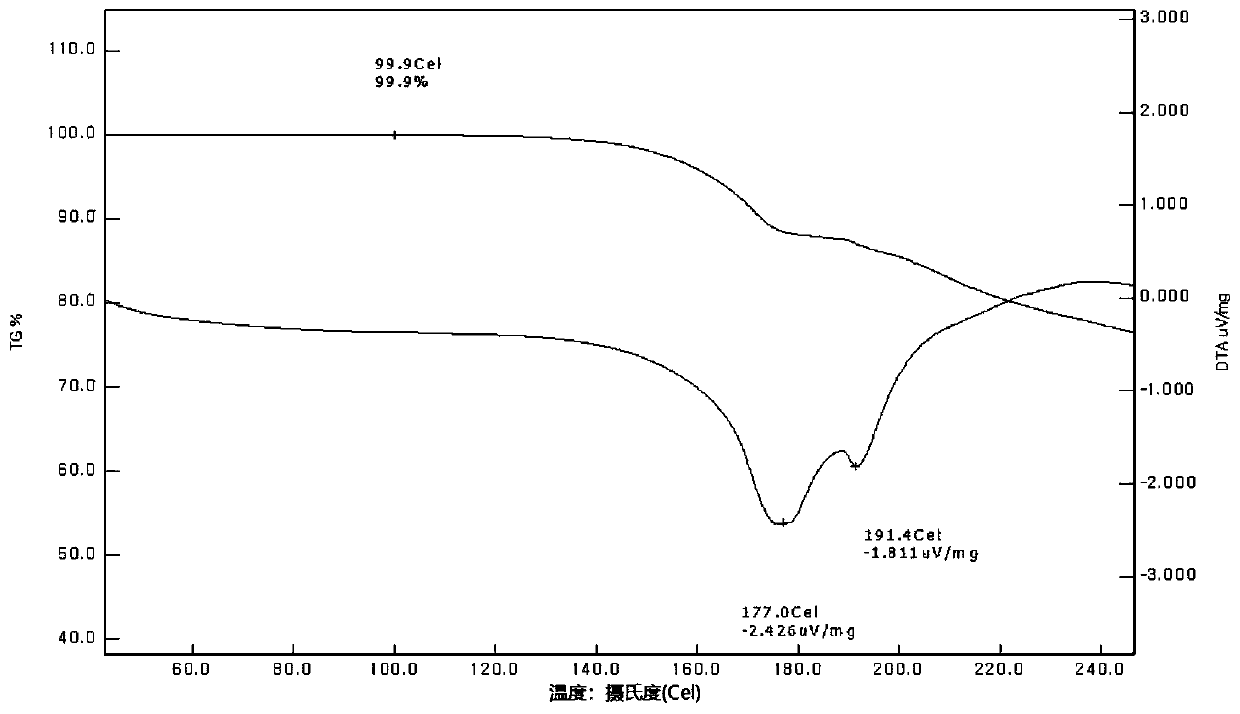
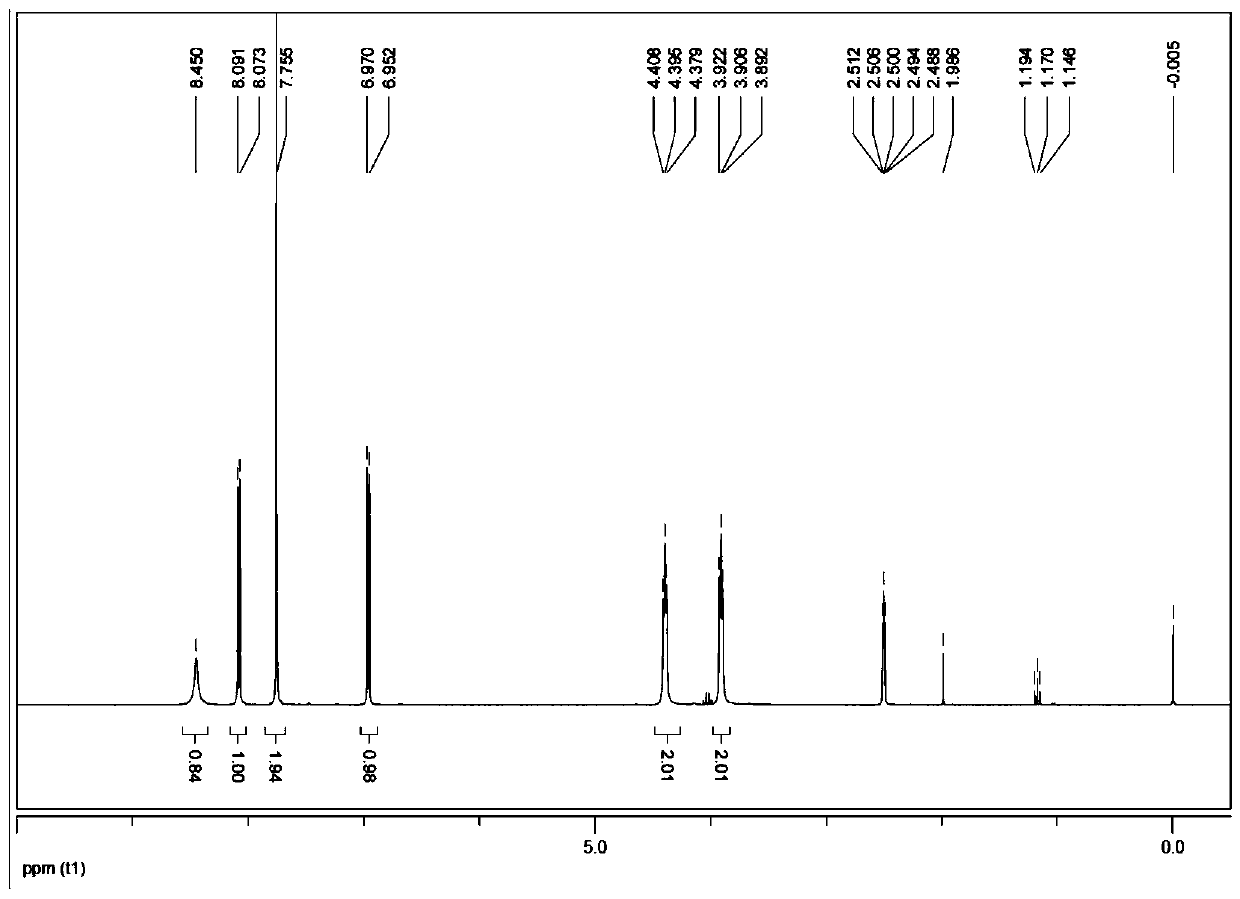
Method for preparing heterocyclic derivative compound, composition containing same compound, and hydrate of same compound
A technology of compounds and hydrates, applied in the direction of drug combinations, organic chemical methods, medical preparations containing active ingredients, etc., can solve the problems of expensive, induced gene mutations, etc., and achieve excellent results in uric acid-related diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0075] The preparation method of the compound of chemical formula I, its salt or hydrate
[0076] The present invention relates to the preparation method of the compound of chemical formula I, its pharmaceutically acceptable salt or its hydrate, comprising the step of coupling the compound of the following chemical formula III with the compound of the following chemical formula IV.
[0077] [reaction formula 1]
[0078]
[0079] In the formula, R is hydrogen or Boc.
[0080] Specifically, a base is added to the compound of the chemical formula III, and the compound 3,4-dihydro-2H-pyrido[4,3-b][1,4]oxazine hydrobromide (2HBr ) for the coupling reaction. The obtained intermediate compound is post-treated to obtain the compound (3,5-dibromo-4-hydroxyl phenyl) (2,3-dihydro-4H-pyrido[4,3-b][ 1,4] oxazin-4-yl)-methanone or a pharmaceutically acceptable salt thereof.
[0081] In a specific embodiment of the present invention, the compound of the chemical formula III can be o...
Embodiment 1
[0167] Synthesis of compound 3,4-dihydro-2H-pyrido[4,3-b][1,4]oxazine hydrobromide (2HBr) of chemical formula IV become
[0168] (1) Preparation of 4-chloro-3-nitropyridine
[0169] 50g (0.356mmol) of 4-hydroxyl-nitropyridine was stirred in 50mL (1T) of DMF (dimethylformamide) and 450mL (9T) of ethyl acetate, and 42.5mL of phosphorus oxychloride ( POCl 3 , 1.3eq), heated up and heated to reflux at 70-80°C for 2 hours. After the reaction, the reaction solution was cooled to 40° C., and 200 mL of water was added to terminate the reaction. The separated organic layer was washed with 200 mL of saturated sodium bicarbonate (NaHCO 3 ), 200 mL of brine (brine), and the collected organic layer was washed with magnesium sulfate (MgSO 4 ) was dried and filtered, then concentrated under reduced pressure to obtain 60 g of light yellow concentrated crystal 4-chloro-3-nitropyridine.
[0170] (2) Preparation of 2-((3-nitropyridin-4-yl)oxy)methyl acetate
[0171] 60g (0.356mmol) of ...
Embodiment 2
[0182] The compound of chemical formula I (3,5-dibromo-4-hydroxyphenyl) (2,3-dihydro-4H-pyrido[4,3-b][1,4]oxa Synthesis of oxazin-4-yl)-methanone
[0183] At 25-30°C, add 1L of tetrahydrofuran (THF) to the reactor, add 139g (0.470mol) of 3,5-dibromo-4-hydroxybenzoic acid, and then add 278g (1.27mol) to the reactor of di-tert-butyl dicarbonate. Add 125g (1.58mol) of pyridine under a nitrogen atmosphere, and stir the reaction solution for 2 hours at 25-30°C to obtain 3,5-dibromo-4-tert-butoxycarbonyloxy-benzoic acid and 3 , The reaction solution of 5-dibromo-4-((tert-butoxycarbonyl)oxy)benzoic acid (tert-butylcarbonyl)anhydride.
[0184] At a temperature of 25 to 30°C, 170 g (1.68 mol) of triethylamine was added to the reaction liquid, and 100 g of 3,4-dihydro-2H prepared in Example 1 was added to the reaction liquid - Pyrido[4,3-b][1,4]oxazine hydrobromide (2HBr), the reaction solution was stirred for 6 hours at a temperature of 25-30°C. Remove the formed salt and collec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com