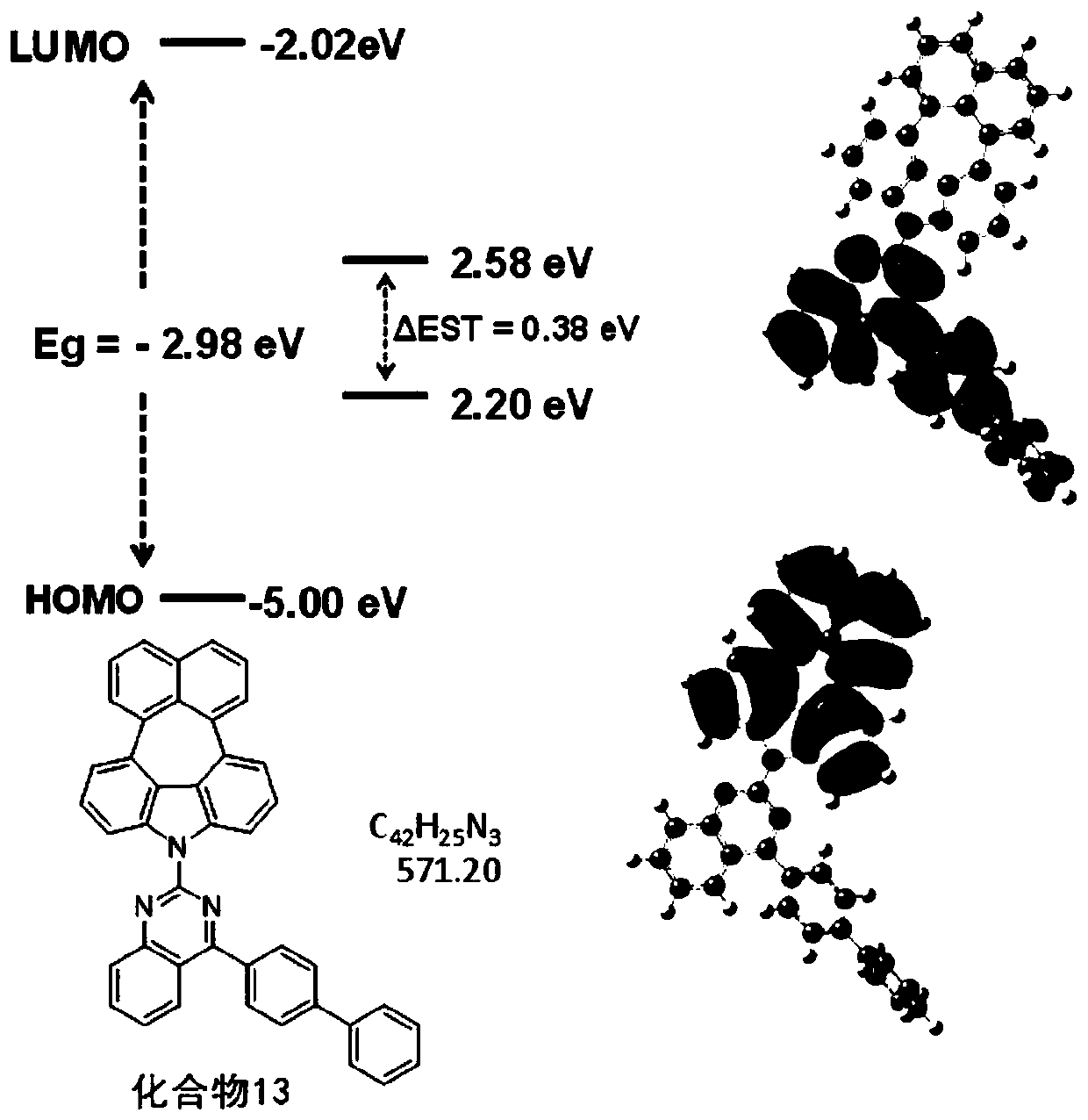
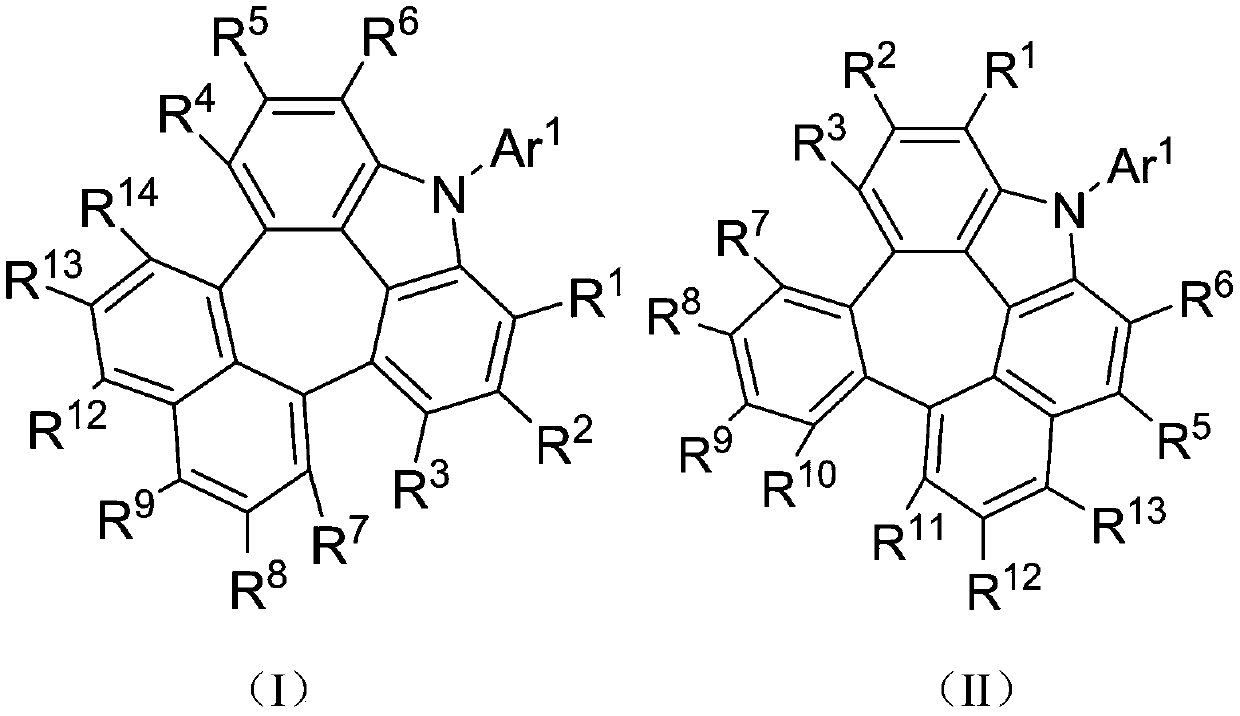
Condensed-ring compound and preparation method and application thereof
A compound and fused ring technology, applied in the field of fused ring compounds and their preparation, can solve the problems of reduced luminous efficiency, shortened service life, and low energy transfer efficiency of devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] This embodiment provides a kind of condensed ring compound (compound 1), has the structure shown in the following formula:
[0084]
[0085] The synthetic route of compound 1 is as follows:
[0086]
[0087] Wherein, the synthetic route of intermediate 5-E is as follows:
[0088]
[0089] The preparation method of compound 1 specifically comprises the following steps:
[0090] (1) Synthesis of intermediate 1-E:
[0091]Get 500 milliliters of double-necked round-bottomed flasks and put into stirrer bar and connect reflux tube, fill with nitrogen after drying; Add compound o-nitrophenylboronic acid (16.70 grams, 1 equivalent), o-bromoiodobenzene (28.19 grams, 1.0 equivalent), potassium carbonate (K 2 CO 3 , 1.5 equivalents), ethanol (50 ml), water (50 ml), toluene (200 ml), tetrakis(triphenylphosphine) palladium (Pd(PPh 3 ) 4 , 0.05 equivalent), then heated to reflux and reacted for 12 hours, cooled to room temperature after the reaction was completed; adde...
Embodiment 2
[0104] This embodiment provides a kind of condensed ring compound (compound 2), has the structure shown in the following formula:
[0105]
[0106] The synthetic route of compound 2 is as follows:
[0107]
[0108] Wherein, the synthetic route of intermediate 5-E' is as follows:
[0109]
[0110] The preparation method of compound 2 specifically comprises the following steps:
[0111] (1) Synthesis of intermediate 1-E':
[0112] The same as the synthetic method of intermediate 1-E, the difference is that 1-bromo-8 iodonaphthalene (31.89 grams, 1 equivalent) is used to replace o-bromoiodobenzene, and the ratio of each raw material is adjusted to obtain intermediate 1-E' (22.56 grams, producing rate 69%)
[0113] (2) Synthesis of intermediate 2-E':
[0114] The same as the synthetic method of intermediate 2-E, the difference is that intermediate 1-E' (32.70 g, 1 equivalent) is used to replace intermediate 1-E, and the ratio of each raw material is adjusted to obtain...
Embodiment 3
[0125] This embodiment provides a condensed ring compound (compound 3), which has the structure shown in the following formula:
[0126]
[0127] The synthetic route of compound 3 is as follows:
[0128]
[0129] Wherein, the synthetic route of intermediate 5-E is the same as that of Example 1.
[0130] The preparation method of compound 3 specifically comprises the following steps:
[0131] (1)-(5) are the same as embodiment 1;
[0132] (6) Synthesis of compound 3:
[0133] The same synthesis method as compound 1, except that compound 1-2 (22.40 g, 1 equivalent) was used instead of compound 1-1, and the ratio of each raw material was adjusted to obtain compound 3 (35.24 g, yield 81%).
[0134] Elemental Analysis: C 30 h 17 N 30 Theoretical: C, 82.74; H, 3.93; N, 9.65; Found: C, 82.75; H, 3.95; N, 9.62; + ): theoretical value: 435.1372; measured value: 435.1377.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com