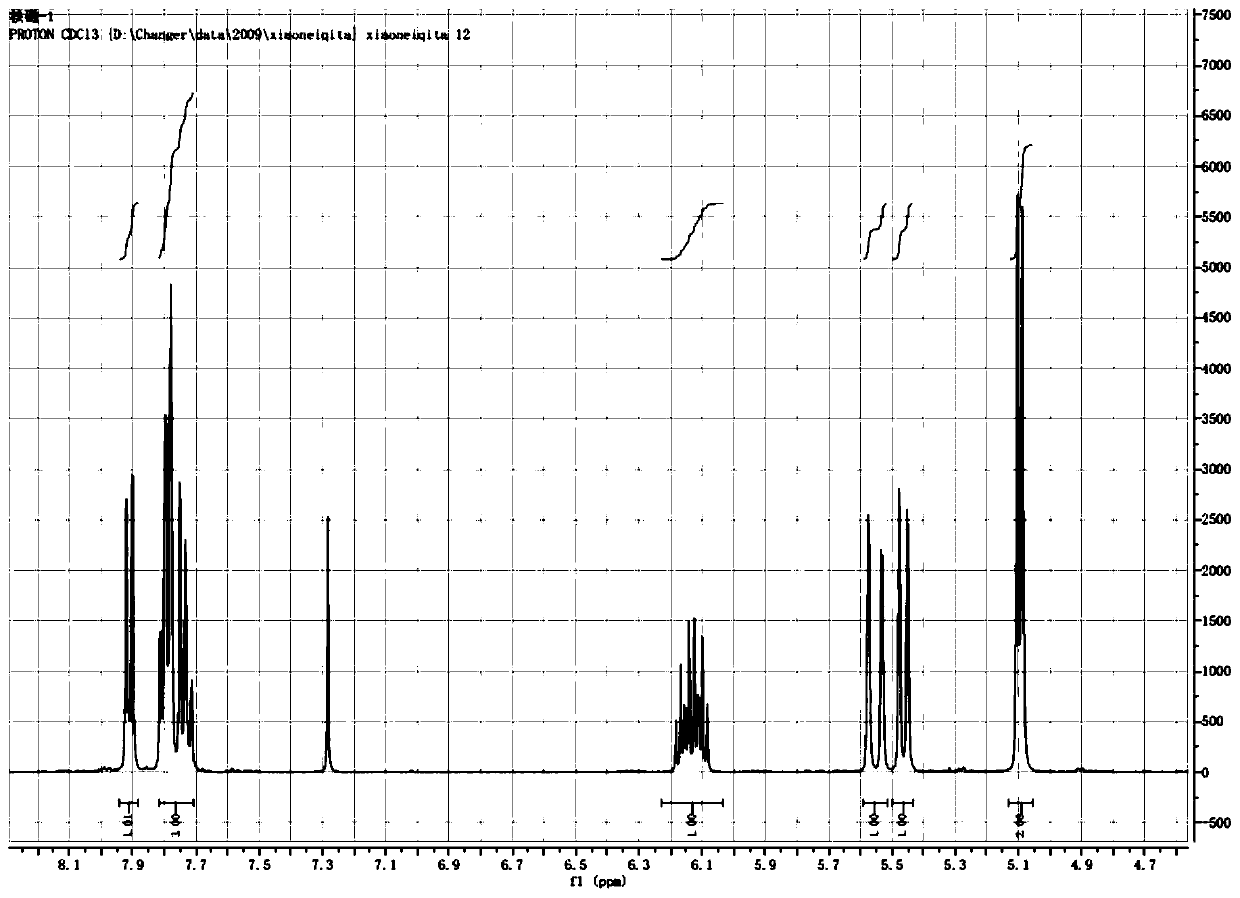
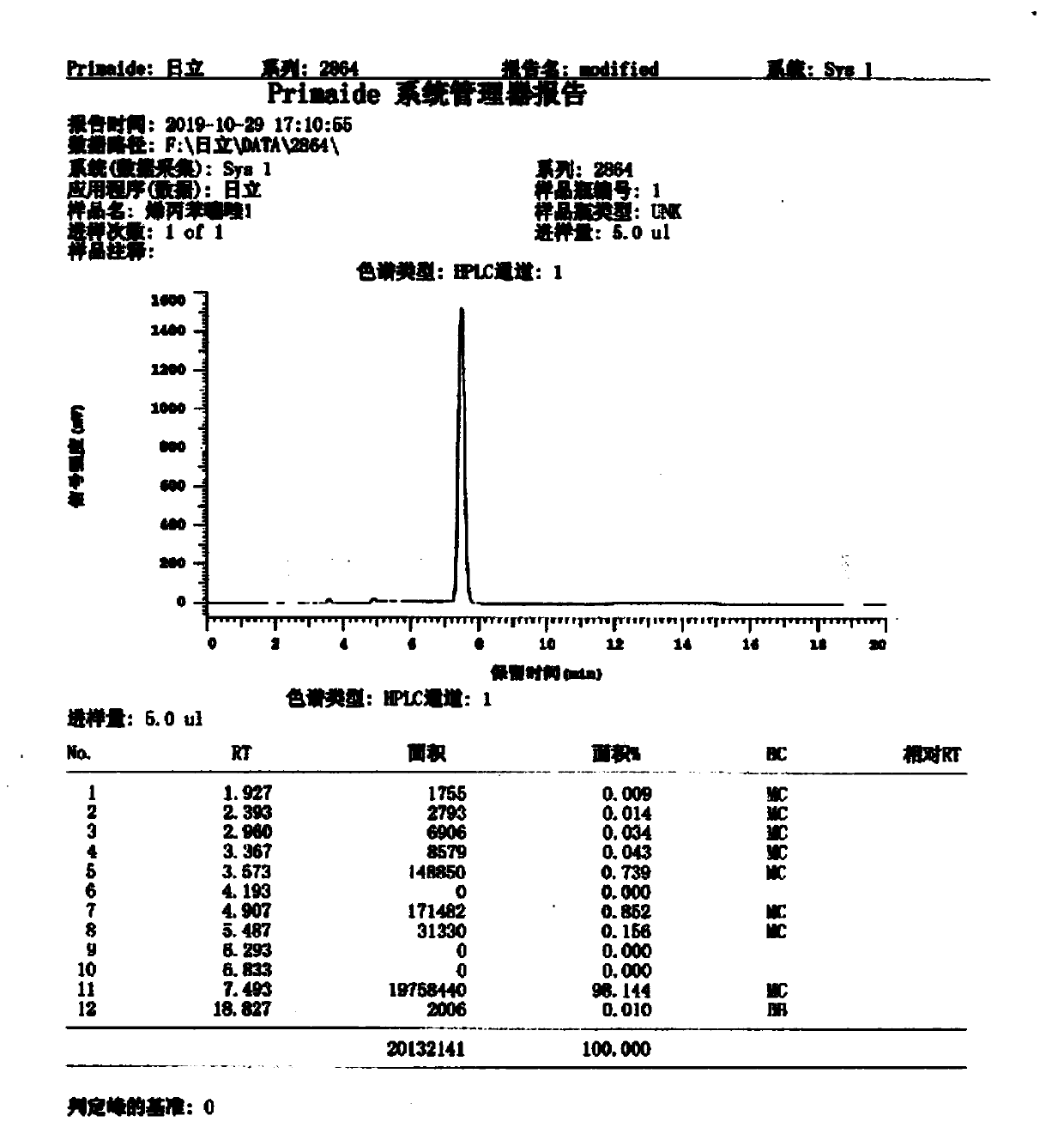
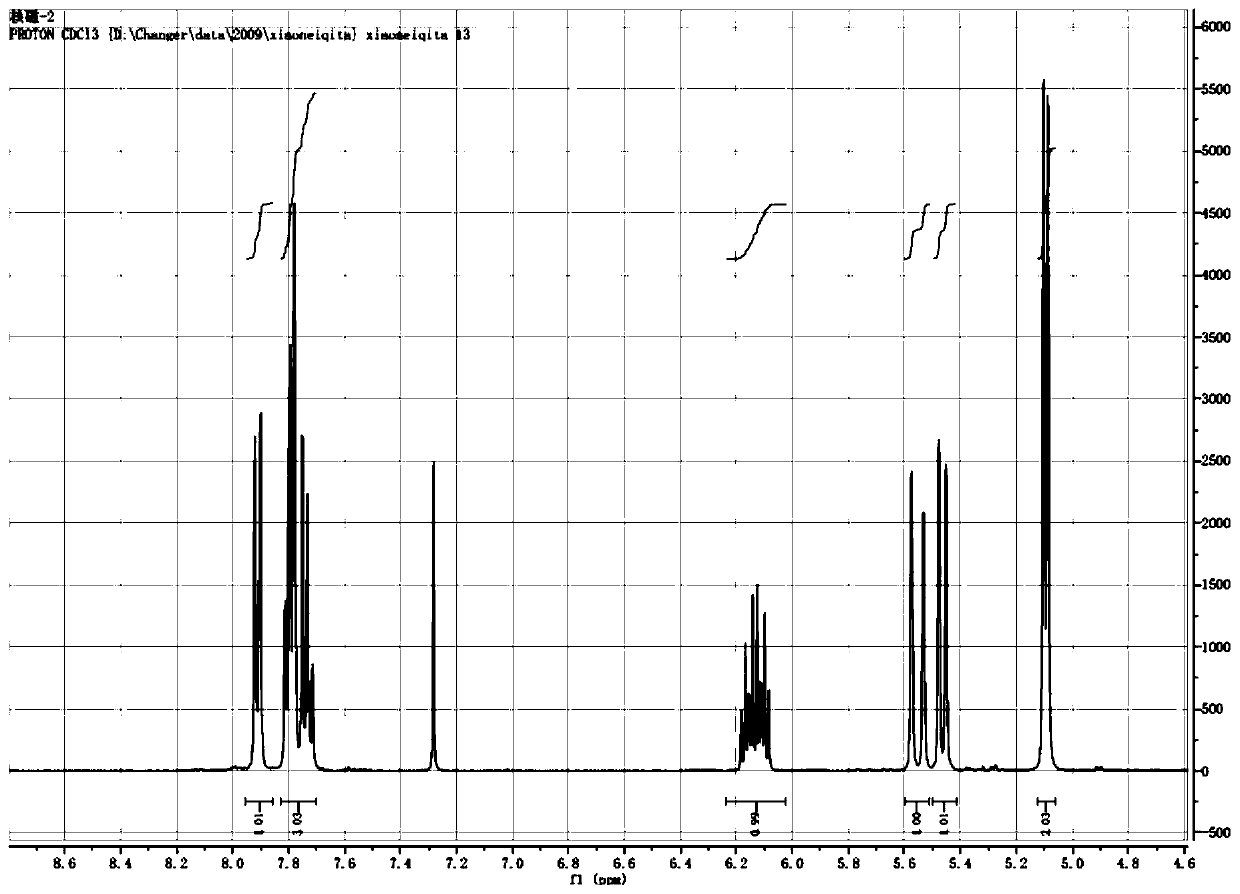
Preparation method of probenazole
A technology of allyl benzothiazole and sodium allyl alkoxide, applied in the direction of organic chemistry, etc., can solve the problems of clogging pipes, increasing the cost of condensation, loss of dichloromethane, damage to the stirring paddle, etc., and achieves easy operation, good price advantage, and reduced The effect of production energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A kind of preparation method of allyl benzothiazole, concrete steps are as follows:
[0033] 1. Chloride preparation:
[0034] (1) Add 500g of thoroughly dried saccharin [FW183, 2.73mol, incomplete drying, consumption of SOCl in a 3L four-necked bottle 2 Increase], 1335g 1,4-dioxane, 552g SOCl 2 【FW119, 4.64mol】, 22g DMF. The upper end of the reflux condenser is sequentially connected to CaCl 2 Drying tower, buffer bottle, hydrogen chloride absorption bottle with 464mL water, buffer bottle, solution of 110g NaOH and 300mL water (used to produce sodium bisulfite or frozen to a liquid state below -10°C for saccharin production).
[0035] (2) Slowly heat to reflux until no gas is released, add 1000g of dry xylene;
[0036] (3) Heating and distilling unreacted thionyl chloride and 1,4-dioxane, and stopping heating when the temperature in the kettle rose to 140° C. to obtain a xylene solution of chloride.
[0037] 2, the preparation of sodium allyl alcoholate:
[0038]...
Embodiment 2
[0042] A kind of preparation method of allyl benzothiazole, concrete steps are as follows:
[0043] 1. Chloride preparation:
[0044] (1) Add 500g of thoroughly dried saccharin [FW183, 2.73mol, incomplete drying, consumption of SOCl in a 3L four-necked bottle 2 Increase], 1335g 1,4-dioxane, 552g SOCl 2 【FW119, 4.64mol】, 22g DMF. The upper end of the reflux condenser is sequentially connected to CaCl 2 Drying tower, buffer bottle, hydrogen chloride absorption bottle with 464mL water, buffer bottle, solution of 110g NaOH and 300mL water (used to produce sodium bisulfite or frozen to a liquid state below -10°C for saccharin production).
[0045] (2) Slowly heat to reflux, until no gas is released, add 1000g of dry chlorobenzene;
[0046] (3) Heating and distilling unreacted thionyl chloride and 1,4-dioxane, and stopping heating when the temperature in the kettle rose to 135° C. to obtain a chlorobenzene solution of chloride.
[0047] 2, the preparation of potassium allyl alc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com