Pd/MnO2-Ni electrode as well as preparation method and application thereof
An electrode and reaction technology, applied in the field of electrochemistry, can solve the problems of high price of active component metal Pd, etc., achieve the effects of reducing preparation cost and use cost, mild conditions, and activity improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
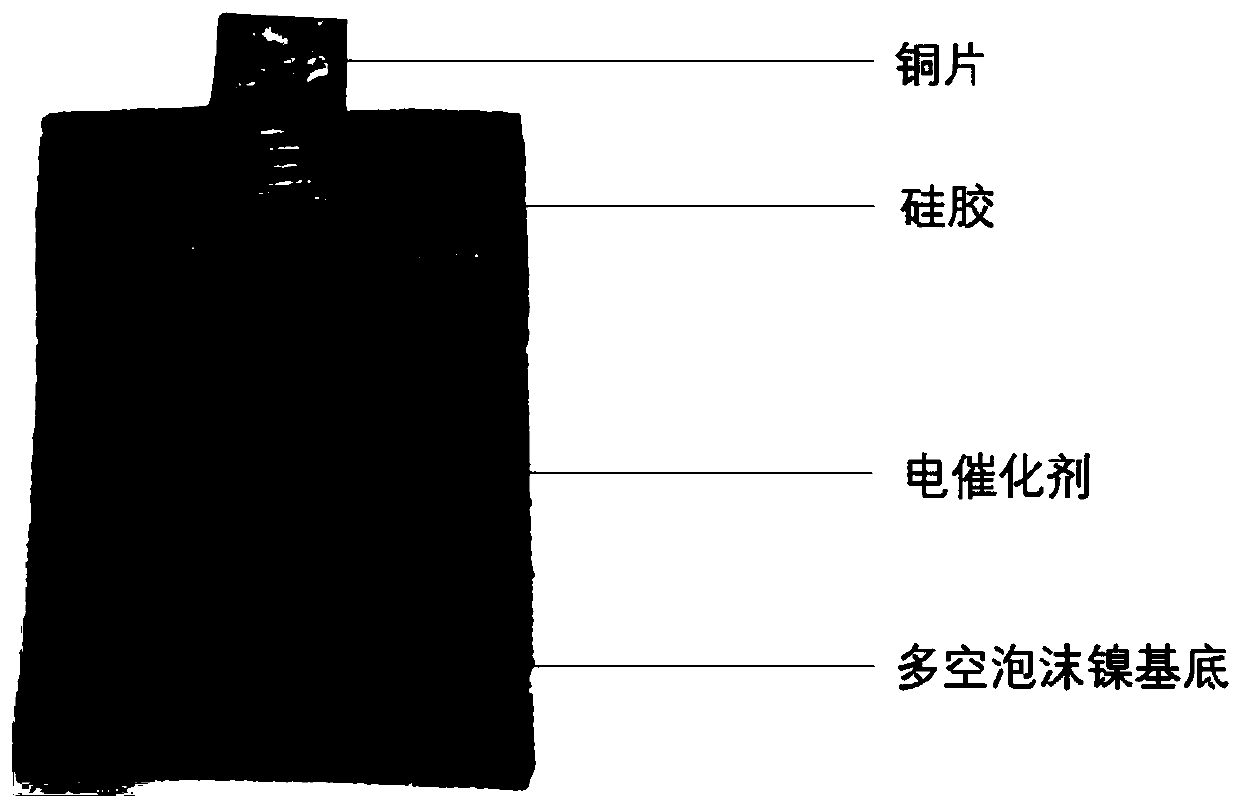
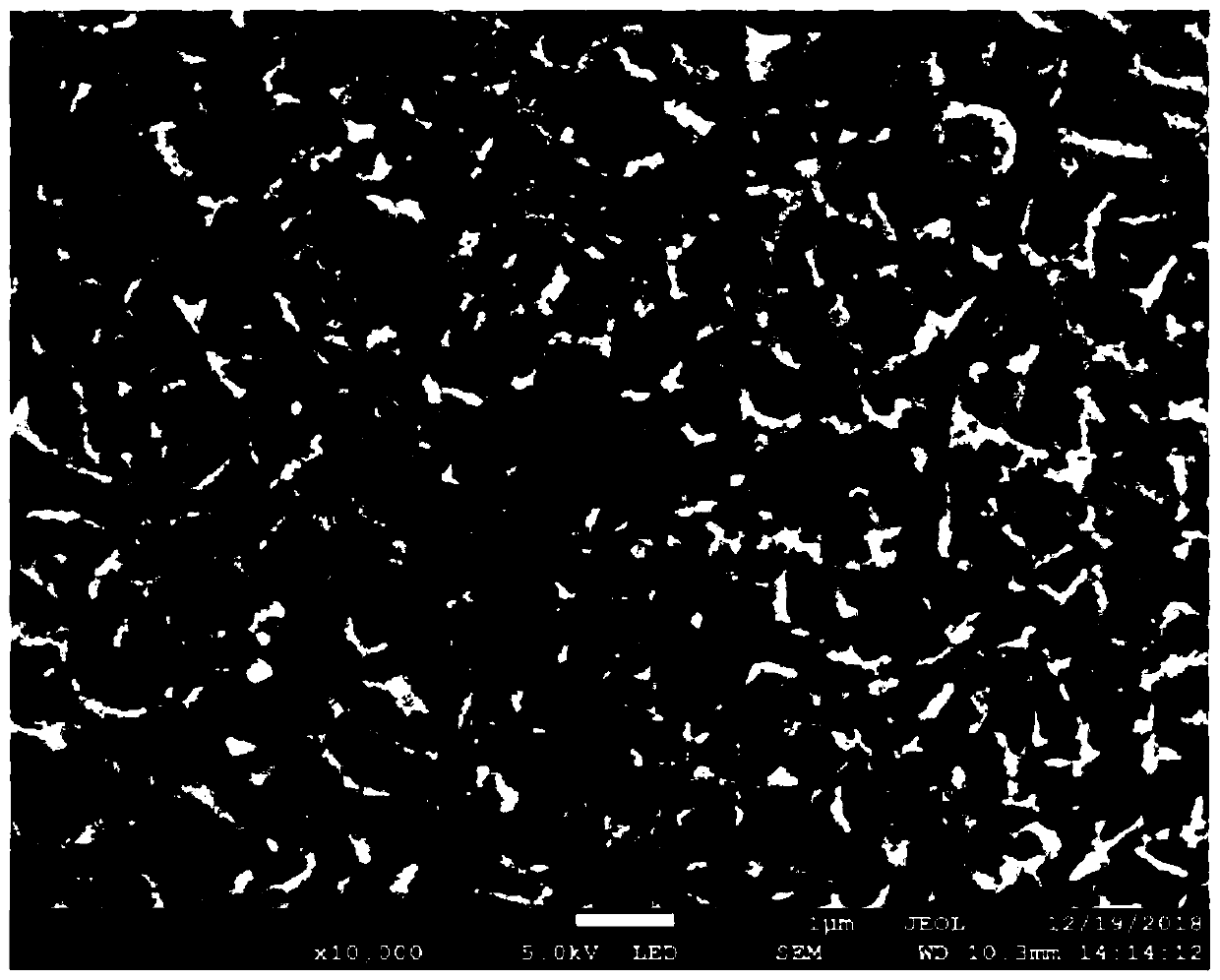
[0032] Embodiment 1: Preparation of Pd / MnO 2 -Ni electrode
[0033] (1) Weigh 250mg of potassium permanganate, disperse it in 50mL of deionized water, put it into an ultrasonic cleaner and ultrasonically homogenize it, then transfer it to a constant temperature magnetic stirrer for stirring;
[0034] (2) Cut porous nickel foam of 4*3cm, immerse in 30mL hydrochloric acid, put it into an ultrasonic cleaner for ultrasonic cleaning for 3 minutes, then take it out and rinse it with deionized water until the eluate is neutral;
[0035] (3) The porous nickel foam obtained in step (2) is transferred to a tetrafluoroethylene hydrothermal kettle with a volume of 100mL, then the solution obtained in step (1) is slowly added to the polytetrafluoroethylene hydrothermal kettle, and finally the hydrothermal The kettle was placed in a constant temperature drying oven at 150°C for 24 hours;
[0036] (4) Rinse the electrode obtained in step (3) with deionized water for 3 times, then place the...
Embodiment 2
[0050] Embodiment 2: dechlorination effect experiment
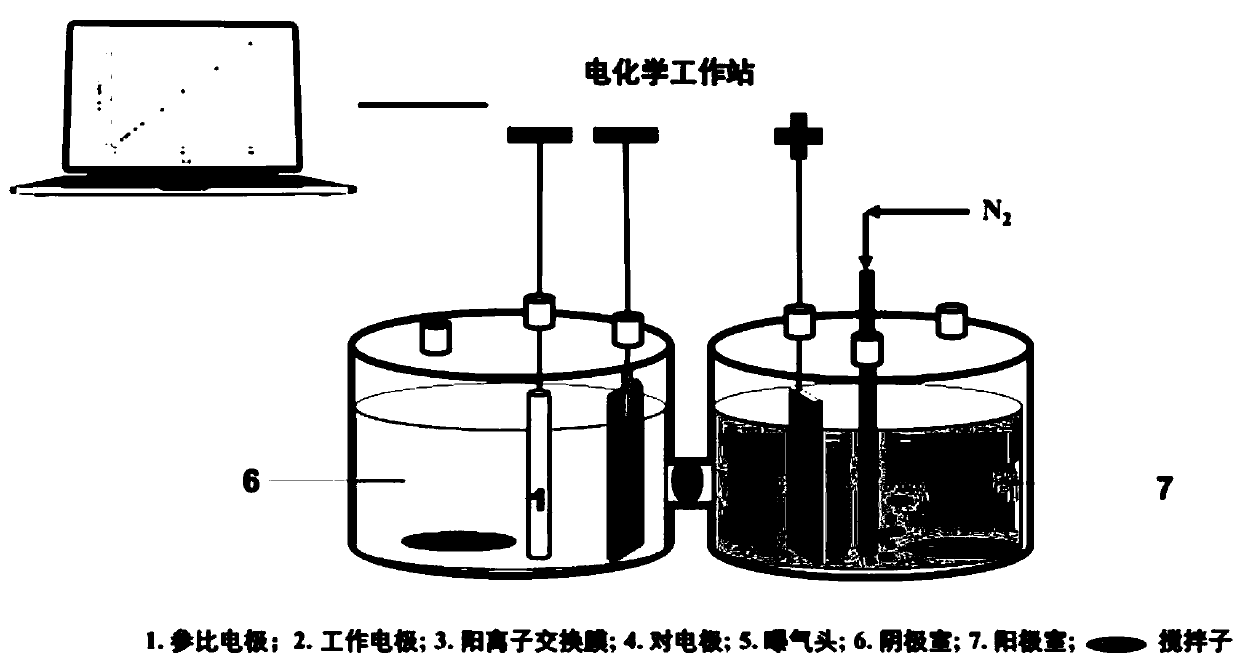
[0051] (1) The construction of the dechlorination reaction device, such as image 3 As shown, the steps are as follows:
[0052] a) The electrolytic cell is an H-type electrolytic cell. The anode chamber and the cathode chamber are separated by a cation exchange membrane (Nafion-117). The volume of the anode chamber and the cathode chamber is 150mL. Add sodium sulfate electrolyte (50mM) to the anode chamber and the cathode chamber, and the volume is 100mL; The electrolyte solution in both the cathode chamber and the cathode chamber needs to be passed through nitrogen for 5 minutes; then add 2,4-dichlorophenol stock solution to the cathode electrolysis chamber with a pipette to make the initial concentration 50mg / L, and then add type B Stirring with magnetic stirring bar;
[0053] b) According to the principle of the three-electrode system, build the circuit of the electrocatalytic dechlorination device. The counter el...
Embodiment 3
[0064] Embodiment 3: Dechlorination effect experiment under different voltages
[0065] Use the Pd / MnO that embodiment 1 prepares 2 -Ni electrode is used as working electrode, carries out dechlorination reaction according to the step of embodiment 2, changes the condition of voltage setting in addition, the value of voltage is respectively set to-0.65,-0.70,-0.75,-0.80,-0.85,-0.90 and -0.95V, carry out 7 dechlorination reactions under the same conditions.
[0066] Pd / MnO at different voltages 2 -Ni electrode dechlorination reaction results are shown in Table 1. As the voltage increases, the amount of active hydrogen species increases continuously, and more active hydrogen is used for dechlorination reaction, so the dechlorination efficiency is continuously enhanced. When the voltage reaches -0.80V, the dechlorination efficiency reaches 100% in the shortest time. However, as the voltage is further increased, although the active hydrogen increases, the amount of hydrogen pro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com