A kind of preparation method of p-methylthiobenzaldehyde
A technology for p-chlorobenzaldehyde and benzaldehyde, which is applied in sulfide preparation, mercaptan preparation, organic chemistry and other directions, can solve the problems of increasing the difficulty of wastewater treatment, a large number of organic impurities, poor operating conditions, etc., and achieves high product yield, The effect of high total yield and good industrialization prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
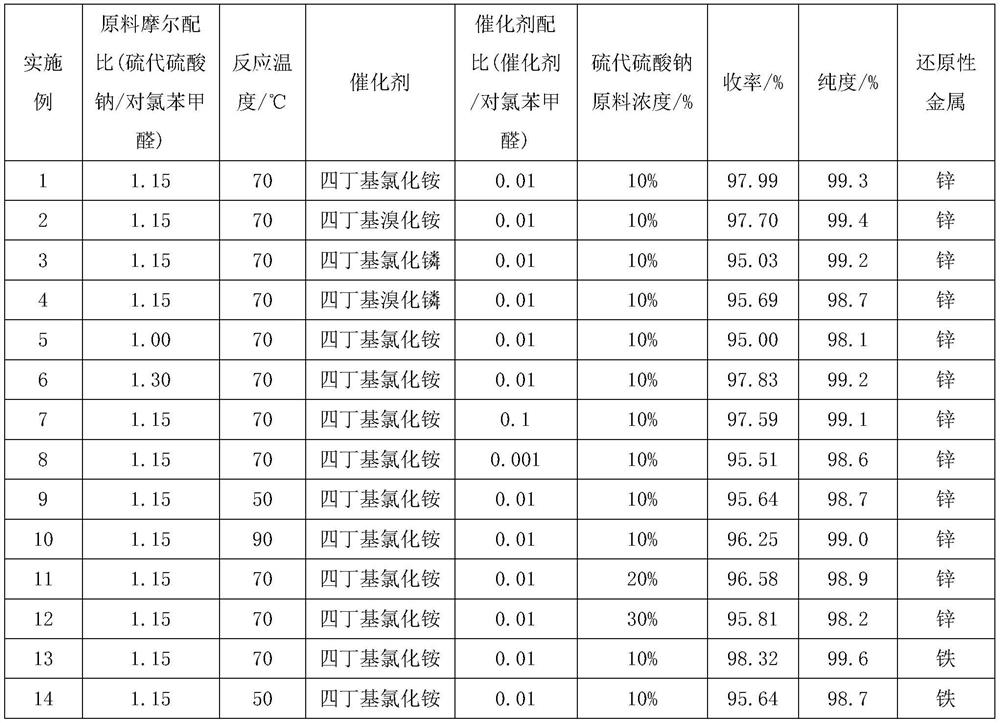
[0028] a. Take 412g (0.261mol) of 10% sodium thiosulfate solution and add it to a four-necked flask with mechanical stirring, add 0.63g (0.00227mol) tetrabutylammonium chloride, raise the temperature to 70°C, and throw in 31.95 g (0.227mol) p-chlorobenzaldehyde, reacted for 4 hours;
[0029] b. Add concentrated sulfuric acid to adjust pH=0, heat up to 90°C, and keep warm for 2 hours; then lower the temperature to 60°C, add 0.15g zinc powder, stir and keep warm for 2 hours; then add 32% liquid alkali dropwise to adjust pH=9.0, Stir for 1 hour;
[0030] c. Put the material into the autoclave, raise the temperature to 90°C, feed in methyl chloride, keep the pressure in the kettle at 0.5MPa, and keep it warm for 8 hours; after the heat preservation is over, cool it to 35°C, and put the material into the separatory funnel to stand still After 30 minutes, the lower organic phase was collected to obtain the product crude product; 31.95g sodium sulfate was added to absorb water in th...
Embodiment 2
[0032] The difference between this implementation method and Example 1 is that the phase transfer catalyst is the tetrabutylammonium bromide of 0.73g (0.00226mol), and other steps are identical, obtain 33.79g product, molar yield 97.70%, gas phase detection purity is 99.3%.
Embodiment 3
[0034] The difference between this implementation method and Example 1 is that the phase transfer catalyst is 0.67g (0.00227mol) tetrabutyl chloride Other steps are the same, 32.86g product is obtained, the molar yield is 95.03%, and the gas phase detection purity is 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com