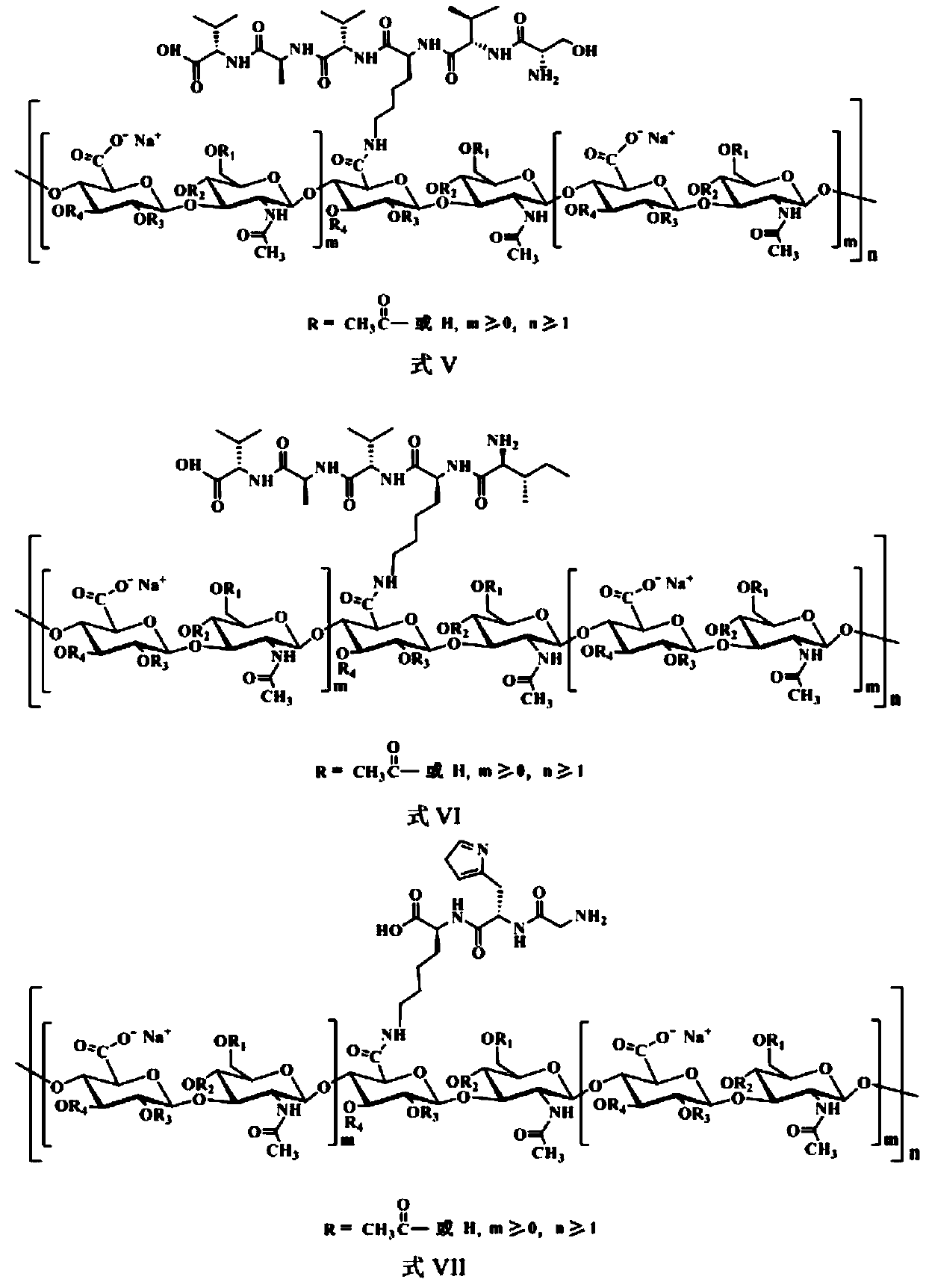
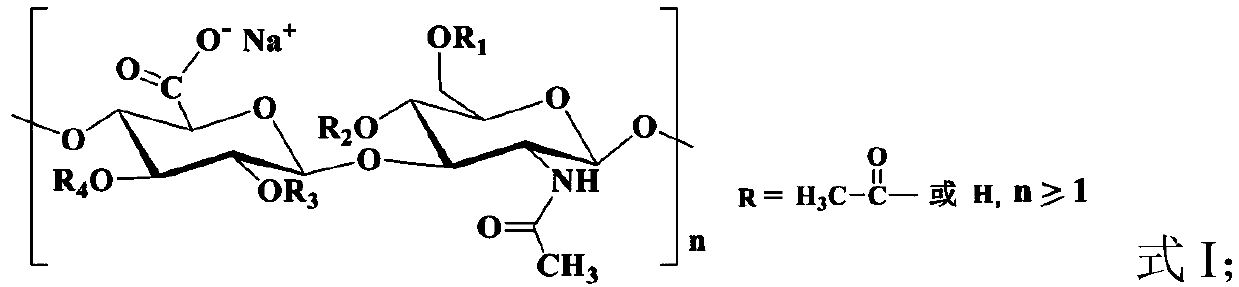
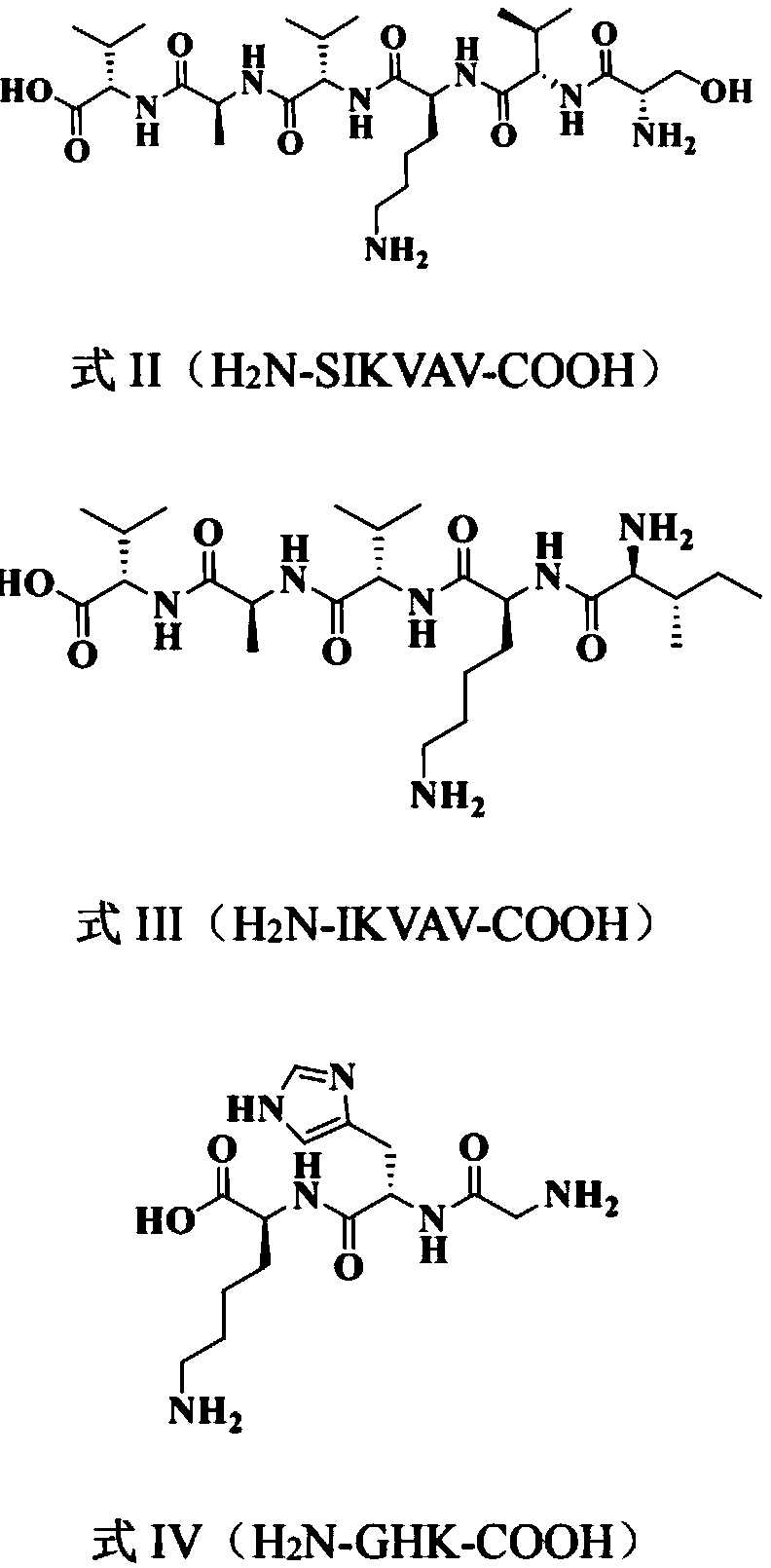
Acetylated hyaluronate oligopeptide and preparation and application methods therefor
The technology of hyaluronan and uronic acid oligopeptide is applied in the field of functional cosmetics, which can solve the problems of hyaluronic acid and oligopeptide being easily inactivated by enzymatic hydrolysis, unstable structure of polypeptide substances, and short activity time. Achieve the effect of promoting penetration of the skin barrier, enhancing moisturizing, wrinkle-removing and anti-aging effects, and being less susceptible to environmental influences
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of hyaluronan-TBA:
[0038] (1) 300g molecular weight is that the hyaluronan of 50kDa is dissolved in 10L deionized water, then passes through 732 (H + ) type cation exchange column to remove the Na + Convert to H + , to obtain an eluent containing hyaluronan;
[0039] (2) Add 200g of TBAOH to the eluent containing hyaluronan in step (1), stir for 2 hours, and neutralize to obtain a hyaluronan-TBA reaction solution;
[0040] (3) ultrafiltration, washing, and concentration of the hyaluronan-TBA reaction solution obtained in step (2) through an ultrafiltration membrane (molecular weight cut-off 500D), to remove excess TBAOH, to obtain a hyaluronan-TBA product;
[0041] (4) Freeze-drying the hyaluronan-TBA product obtained in step (3) to obtain a hyaluronan-TBA solid powder.
Embodiment 2
[0043] Preparation of Fmoc-Ser-Ile-Lys-Val-Ala-Val-COOH crude peptide:
[0044] (1) Resin swelling: Take 25g of CTC resin with a substitution degree of 1.2mmol / g, add 200ml DCM to swell the resin for 0.5h, drain the solvent, wash the resin twice with DMF, and drain the solvent;
[0045] (2) Preparation of Fmoc-Val-CTC resin: a) Mix Fmoc-Val-OH, DIEA and the CTC resin swollen in step (1) according to the ratio of molar mass ratio of 3:6:1, at 25°C Reaction under conditions for 2 hours to obtain Fmoc-Val-CTC resin; b) Add the mixed solution of MeOH, DMF and DIEA to the resin, react at 30°C for 30 minutes, seal the resin, and wash the resin twice with DMF, Drain the solvent to obtain the blocked Fmoc-Val-CTC resin;
[0046] (3) Removal of Fmoc protecting group: add volume fraction 20% PIP-DMF solution to the blocked Fmoc-Val-CTC resin obtained in step (2), and carry out de-Fmoc protection twice under the condition of 10~30°C : After the first de-Fmoc protection and the second d...
Embodiment 3
[0052] Preparation of Fmoc-Ile-Lys-Val-Ala-Val-COOH crude peptide:
[0053] (1) Resin swelling: Take 25g of CTC resin with a substitution degree of 1.2mmol / g, add 200ml DCM to swell the resin for 0.5h, drain the solvent, wash the resin twice with DMF, and drain the solvent;
[0054] (2) Preparation of Fmoc-Val-CTC resin: a) Mix Fmoc-Val-OH, DIEA and resin according to the molar mass ratio of 3:6:1, react at 25°C for 2h to obtain Fmoc-Val- Val-CTC resin; b) Add the mixed solution of MeOH, DMF and DIEA to the resin, react at 10-30°C for 30 minutes, seal the resin, wash the resin twice with DMF, and drain the solvent to obtain the seal After Fmoc-Val-CTC resin;
[0055] (3) Removal of Fmoc protecting group: add volume fraction 20% PIP-DMF solution to the blocked Fmoc-Val-CTC resin obtained in step (2), and carry out de-Fmoc protection twice under the condition of 10~30°C : After the first de-Fmoc protection and the second de-Fmoc protection, wash the resin with DMF until the pH...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com