Binuclear ionic liquid type heteropolyacid salt solid acid catalyst for synthesizing chalcone derivatives, preparation method and application
A technology of solid acid catalyst and ionic liquid, which is applied in the field of preparation of green heterogeneous catalysts and achieves the effects of good recovery and reuse performance, high activity and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
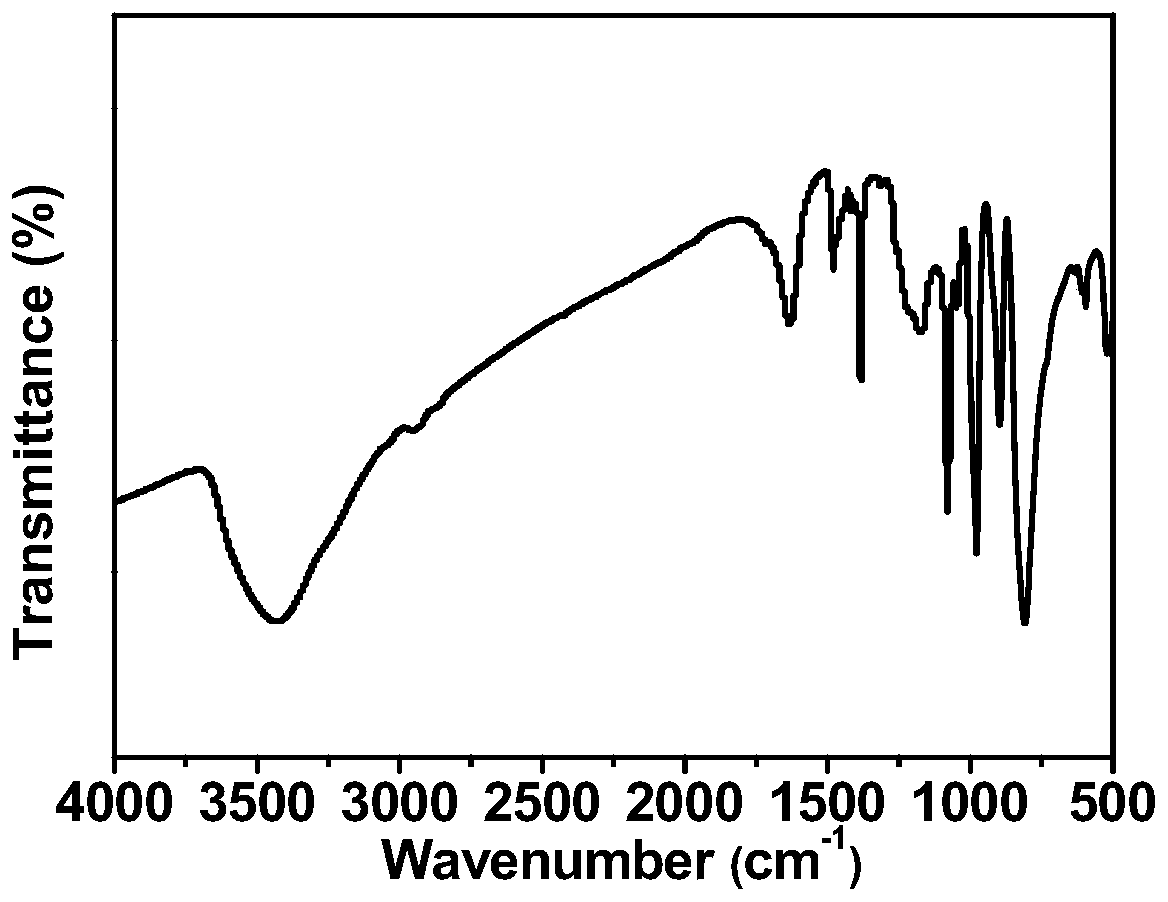
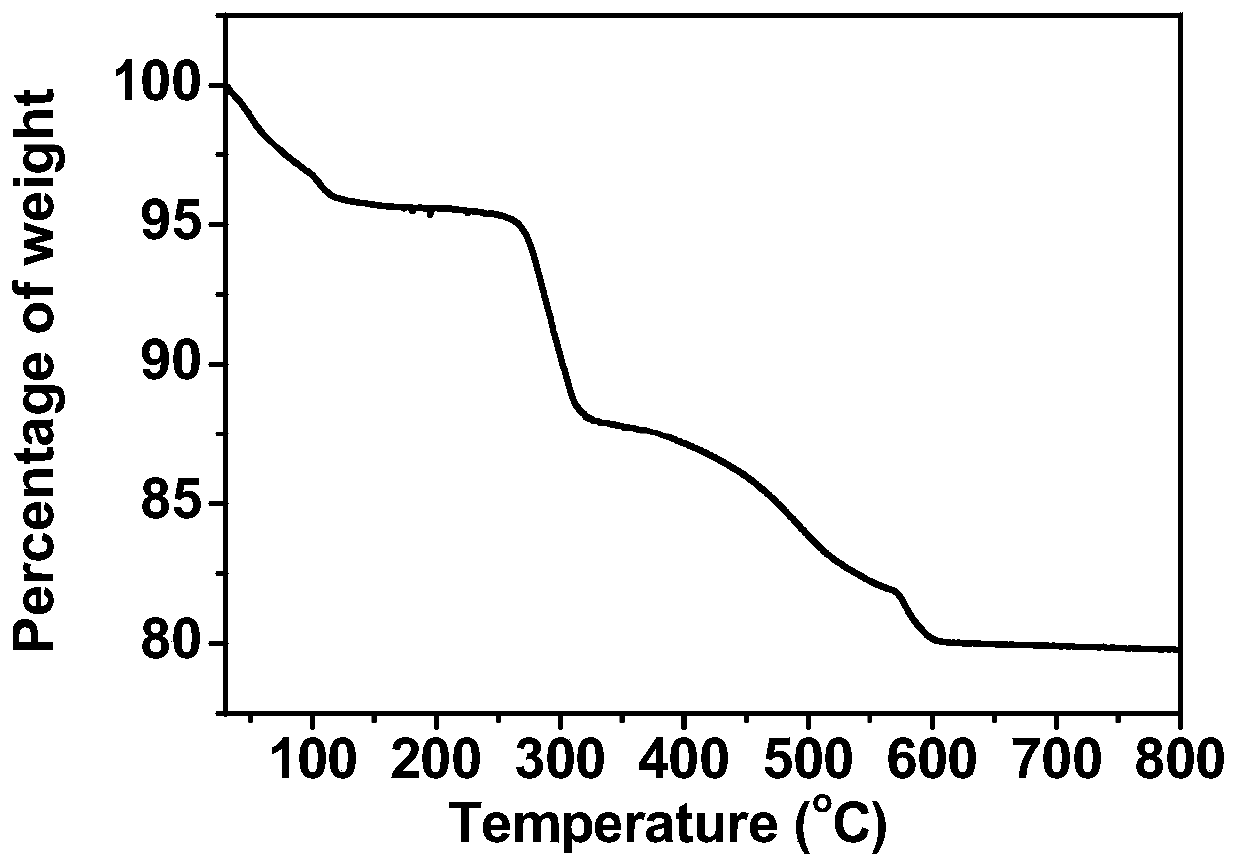
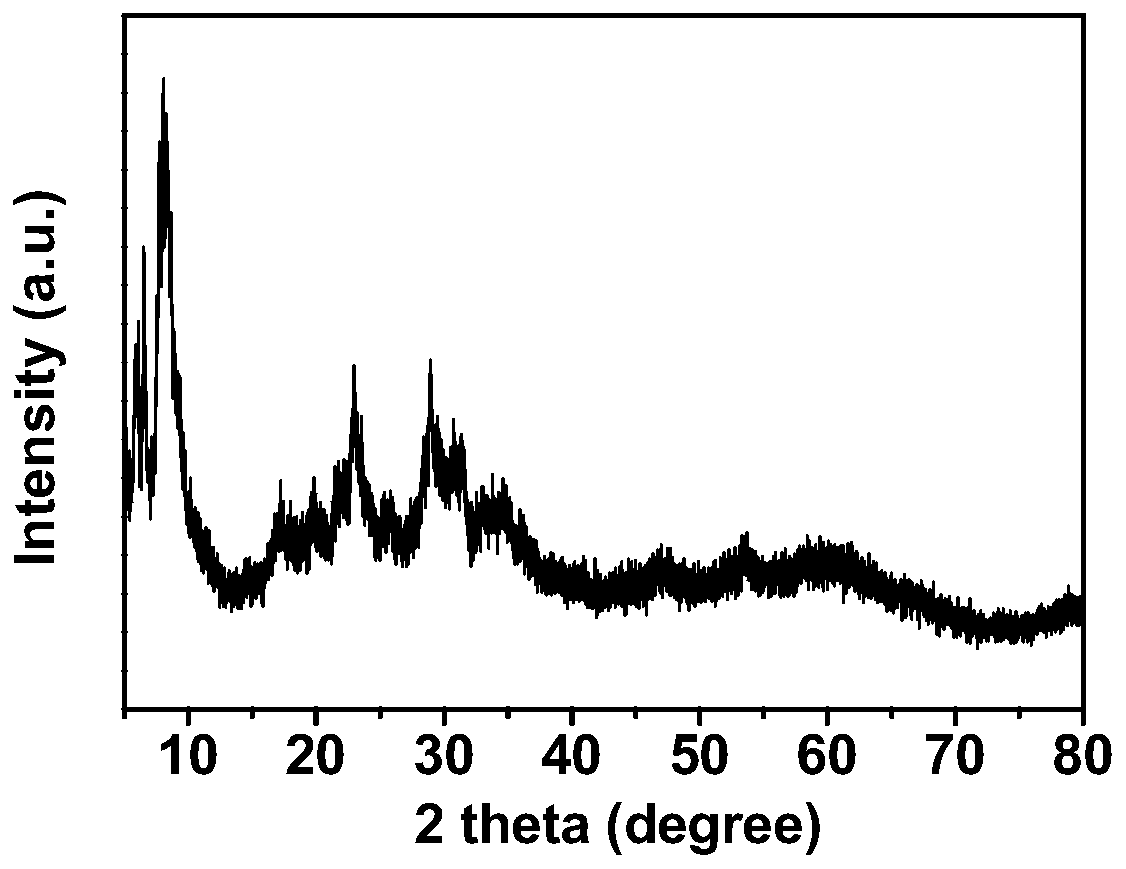
[0037] 1) Synthesis of sulfonic acid functionalized binuclear ionic liquid inner salt THDAPS
[0038] Dissolve tetramethylhexamethylenediamine (4.3g, 25mmol) in 20mL of acetonitrile, and slowly add 1,3-propane sultone (6.1g, 50mmol) dropwise under magnetic stirring and ice-bath conditions. After 12 hours of reaction, A blocky white crude product was obtained, which was washed three times with ether and ethyl acetate respectively to obtain the pure inner salt THDAPS.
[0039] 2) Binuclear ionic liquid type heteropoly salt solid acid catalyst [THDAPS] 1.5 Synthesis of PW
[0040]The above inner salt [THDAPS] (1.0g, 2.4mmol) and phosphotungstic acid (4.6g, 1.6mmol) were dissolved in 20mL of deionized water respectively, and then the aqueous solution of phosphotungstic acid was added dropwise to the inner salt solution, at room temperature The reaction was carried out under magnetic stirring for 24 hours. After the reaction was completed, the resulting suspension solution was pl...
Embodiment 2
[0046] 1) Synthesis of sulfonic acid functionalized binuclear ionic liquid inner salt TEDAPS
[0047] Dissolve tetramethylethylenediamine (2.9g, 25mmol) in 40mL of acetonitrile, and slowly add 1,3-propane sultone (9.2g, 75mmol) dropwise under magnetic stirring and ice bath conditions. After 16 hours of reaction, A blocky white crude product was obtained, which was washed three times with ether and ethyl acetate respectively to obtain the pure inner salt TEDAPS.
[0048] 2) Binuclear ionic liquid type heteropoly salt solid acid catalyst [TEDAPS] 1.5 Synthesis of PMo
[0049] The above-mentioned inner salt [TEDAPS] (1.0g, 2.8mmol) and phosphomolybdic acid (5.11g, 2.8mmol) were dissolved in 30mL deionized water, and then the aqueous solution of phosphomolybdic acid was added dropwise to the inner salt solution, at room temperature The reaction was carried out under magnetic stirring for 18 hours. After the reaction was completed, the obtained suspension solution was placed in a...
Embodiment 3
[0052] 1) Synthesis of sulfonic acid functionalized binuclear ionic liquid inner salt TBDAPS
[0053] Dissolve tetramethylbutanediamine (3.6g, 25mmol) in 50mL of acetonitrile, under magnetic stirring and ice bath conditions, slowly add 1,3-propane sultone (7.6g, 62.5mmol) dropwise, and after 20h of reaction , to obtain a blocky white crude product, which was washed three times with ether and ethyl acetate to obtain the pure product inner salt TBDAPS.
[0054] 2) Binuclear ionic liquid type heteropoly acid salt solid acid catalyst [TBDAPS] 2 Synthesis of SiW
[0055] The above inner salt TBDAPS (1.0g, 2.6mmol) and silicotungstic acid (5.18g, 1.8mmol) were dissolved in 15mL of deionized water respectively, then the aqueous solution of silicotungstic acid was added dropwise to the inner salt solution, and magnetically Stir the reaction for 18 hours. After the reaction is completed, place the resulting suspension in an oven at 70°C. After evaporating water to dryness, the white ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com