A novel thiophenedicarboxylic acid-based copolyester and its preparation method and application
A technology of thiophenedicarboxylic acid-based copolyester and thiophenedicarboxylic acid, which is applied in the field of polymers, can solve problems such as limited application range and yellowing of polyester color, and achieve high light transmittance, high gas barrier performance, and high dependent effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] One aspect of the embodiments of the present invention provides a method for preparing a novel thiophenedicarboxylic acid-based copolyester, comprising:
[0025] Under a protective atmosphere, 2,5-thiophenedicarboxylic acid and / or 2,5-thiophenedicarboxylate, 2,4-thiophenedicarboxylic acid and / or 2,4-thiophenedicarboxylate, di- A new type of thiophenedicarboxylic acid-based copolyester is obtained by the reaction of polyalcohol, catalyst, stabilizer and antioxidant.
[0026] Further, the catalysts include esterification catalysts, transesterification catalysts and polycondensation catalysts.
[0027] In some specific embodiments, the preparation method of the novel thiophenedicarboxylic acid-based copolyester specifically includes: under a protective atmosphere, making 2,5-thiophenedicarboxylic acid and / or 2,5-thiophenedicarboxylic acid The mixture of ester compound, 2,4-thiophenedicarboxylic acid and / or 2,4-thiophenedicarboxylate, glycol, esterification catalyst and / or...
Embodiment 1
[0054] Add 0.1 mol of dimethyl 2,5-thiophene dicarboxylate, 0.1 mol of dimethyl 2,4-thiophene dicarboxylate, 0.32 mol of ethylene glycol, and 0.0003 mol of anhydrous zinc acetate into the reactor, pumping Vacuum and nitrogen were replaced three times, start stirring, gradually heat up to 180 ° C, and react for 3 h, then add 0.0003 mol of antimony trioxide, 0.0004 mol of triphenyl phosphate, 0.00025 mol of tetra[β-(3,5-di-tert-butyl] yl-4-hydroxyphenyl)propionic acid] pentaerythritol ester, and then slowly evacuated to below 60Pa, and heated to 220°C, and reacted for 4.5h to obtain polyethylene thiophene dicarboxylate, which is polyethylene thiophene dicarboxylate. ester 1 The data of H-NMR (deuterated trifluoroacetic acid as solvent) are: thiophene ring hydrogen shifts of 7.57 ppm, 7.78 ppm and 8.68 ppm; ethylene glycol methylene hydrogen atom shifts of 4.20 ppm.
[0055] After testing, the relative number average molecular mass of the obtained polyethylene thiophene dicarbox...
Embodiment 2
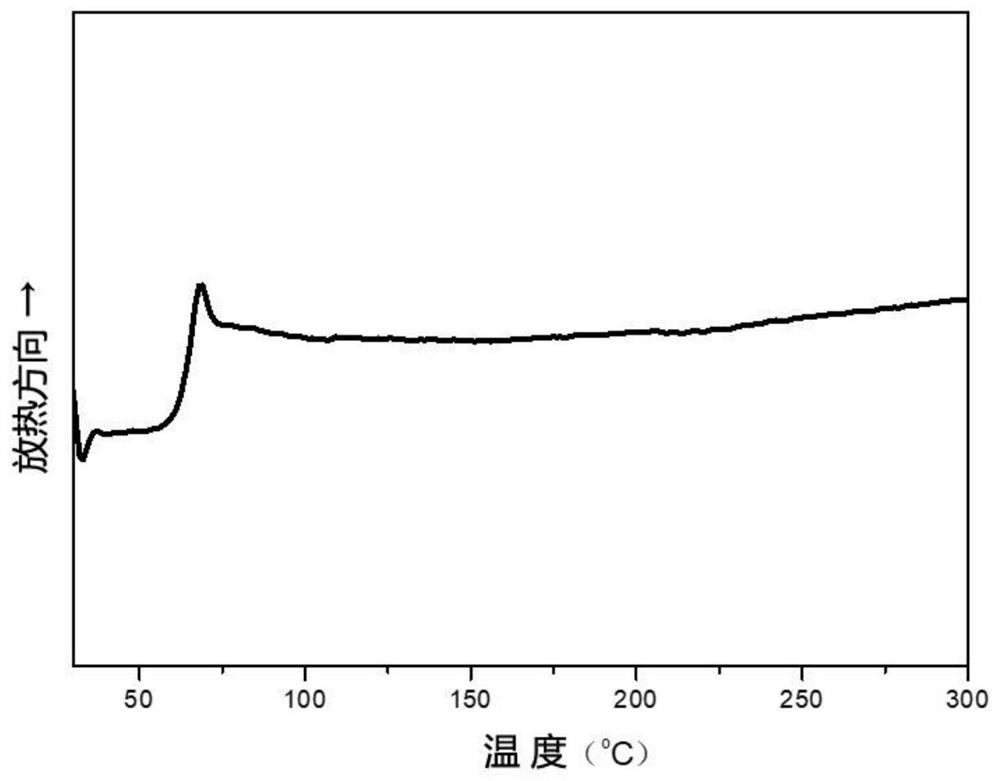
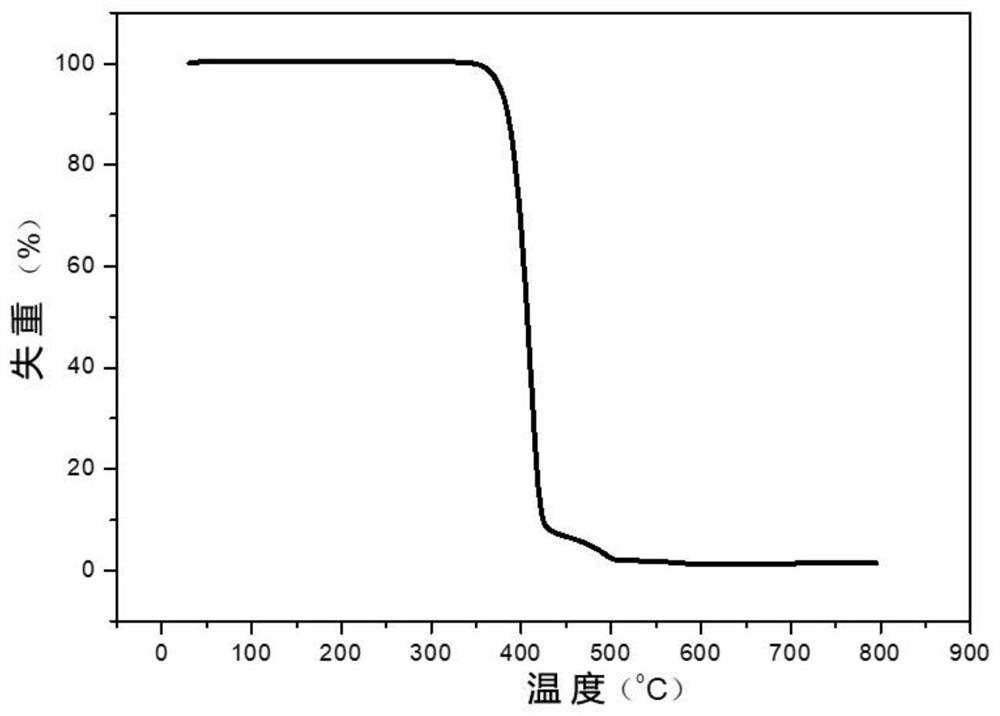
[0057] 0.16mol of dimethyl 2,5-thiophene dicarboxylate, 0.04mol of dimethyl 2,4-thiophene dicarboxylate, 0.32mol of 1,3-propanediol, 0.0004mol of anhydrous zinc acetate were added to the reactor , evacuated and replaced with nitrogen three times, turned on stirring, gradually heated up to 180 ° C, and reacted for 3 h, then added 0.00045 mol of ethylene glycol antimony, 0.0006 mol of trimethyl phosphate, 0.0004 mol of tetrakis[β-(3,5-dimethicone) tert-butyl-4-hydroxyphenyl)propionic acid] pentaerythritol ester, and then slowly vacuumed to below 60Pa, and heated to 210 ° C, and reacted for 3.0h to obtain polythiophene dicarboxylate. After testing, polythiophene dicarboxylate was obtained. The relative number average molecular mass of the ester is 43000g / mol; the visible light transmittance is 89% (cut off 700nm wavelength), the melt viscosity is lower than 720Pa·S at 220℃, and the glass transition temperature is 34.2℃; T in nitrogen 5 % The thermal weight loss temperature is 385...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com