Nitrogen-doped nickel phosphide nano-flower, preparation method and applications thereof
A technology of nickel phosphide and nanoflowers, applied in chemical instruments and methods, phosphides, nanotechnology, etc., to achieve the effect of simple and easy process, high stability and regular shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
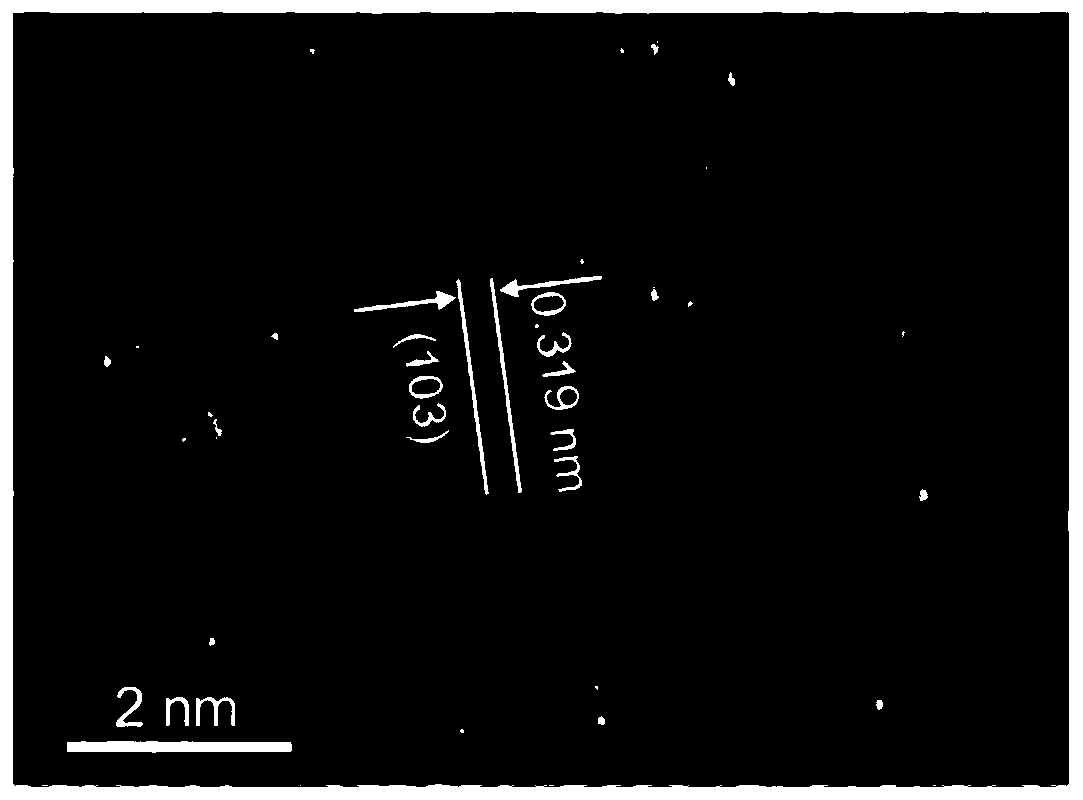
Image
Examples
Embodiment 1
[0037] A preparation method of nitrogen-doped nickel phosphide nanoflowers, comprising the following steps:
[0038] 1)Ni(OH) 2 Preparation of nanoflowers: Dissolve nickel nitrate in absolute ethanol, fully dissolve, add a mixed solution of oleylamine and ethanol during stirring, stir for 30 minutes, transfer to a 50mL reaction kettle, and conduct a hydrothermal reaction in an oven at 180°C for 15 hours. Centrifuged and dried to obtain Ni(OH) 2 nanoflowers;
[0039] 2) Preparation of NiO nanoflowers: Ni(OH) 2 The nanoflowers were placed in a porcelain boat, and in an air atmosphere, the temperature was programmed to rise to 300°C at 2°C / min for heat treatment, and kept at this temperature for 60 minutes, and then cooled to obtain NiO nanoflowers.
[0040] 3) Preparation of nitrogen-doped nickel phosphide nanoflowers: According to the mass ratio of NiO nanoflowers to sodium hypophosphite of 1:30 and the mass ratio of ammonium bicarbonate to 1:2, sodium hypophosphite, bicarbo...
Embodiment 2
[0042] A preparation method of nitrogen-doped nickel phosphide nanoflowers, comprising the following steps:
[0043] 1)Ni(OH) 2 Preparation of nanoflowers: Dissolve nickel nitrate in absolute ethanol, fully dissolve, add a mixed solution of oleylamine and ethanol during stirring, stir for 30 minutes, transfer to a 50mL reaction kettle, and conduct a hydrothermal reaction in an oven at 180°C for 15 hours. Centrifuged and dried to obtain Ni(OH) 2 nanoflowers;
[0044] 2) Preparation of NiO nanoflowers: Ni(OH) 2 The nanoflowers were placed in a porcelain boat, and in an air atmosphere, the temperature was programmed to rise to 300°C at 2°C / min for heat treatment, and kept at this temperature for 60 minutes, and then cooled to obtain NiO nanoflowers.
[0045] 3) Preparation of nitrogen-doped nickel phosphide nanoflowers: According to the mass ratio of NiO nanoflowers to sodium hypophosphite of 1:30 and the mass ratio of ammonium bicarbonate to 1:1, sodium hypophosphite, bicarbo...
Embodiment 3
[0047] A preparation method of nitrogen-doped nickel phosphide nanoflowers, comprising the following steps:
[0048] 1)Ni(OH) 2 Preparation of nanoflowers: Dissolve nickel nitrate in absolute ethanol, fully dissolve, add oleylamine and ethanol mixed solution during stirring, stir for 30min, transfer to a 50mL reaction kettle, and conduct a hydrothermal reaction in an oven at 180°C for 15h. Centrifuged and dried to obtain Ni(OH) 2 nanoflowers;
[0049] 2) Preparation of NiO nanoflowers: Ni(OH) 2The nanoflowers were placed in a porcelain boat, and in an air atmosphere, the temperature was programmed to rise to 300°C at 2°C / min for heat treatment, and kept at this temperature for 60 minutes, and then cooled to obtain NiO nanoflowers.
[0050] 3) Preparation of nitrogen-doped nickel phosphide nanoflowers: According to the mass ratio of NiO nanoflowers to sodium hypophosphite of 1:30 and the mass ratio of ammonium bicarbonate to 1:5, sodium hypophosphite, bicarbonate Ammonium a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com