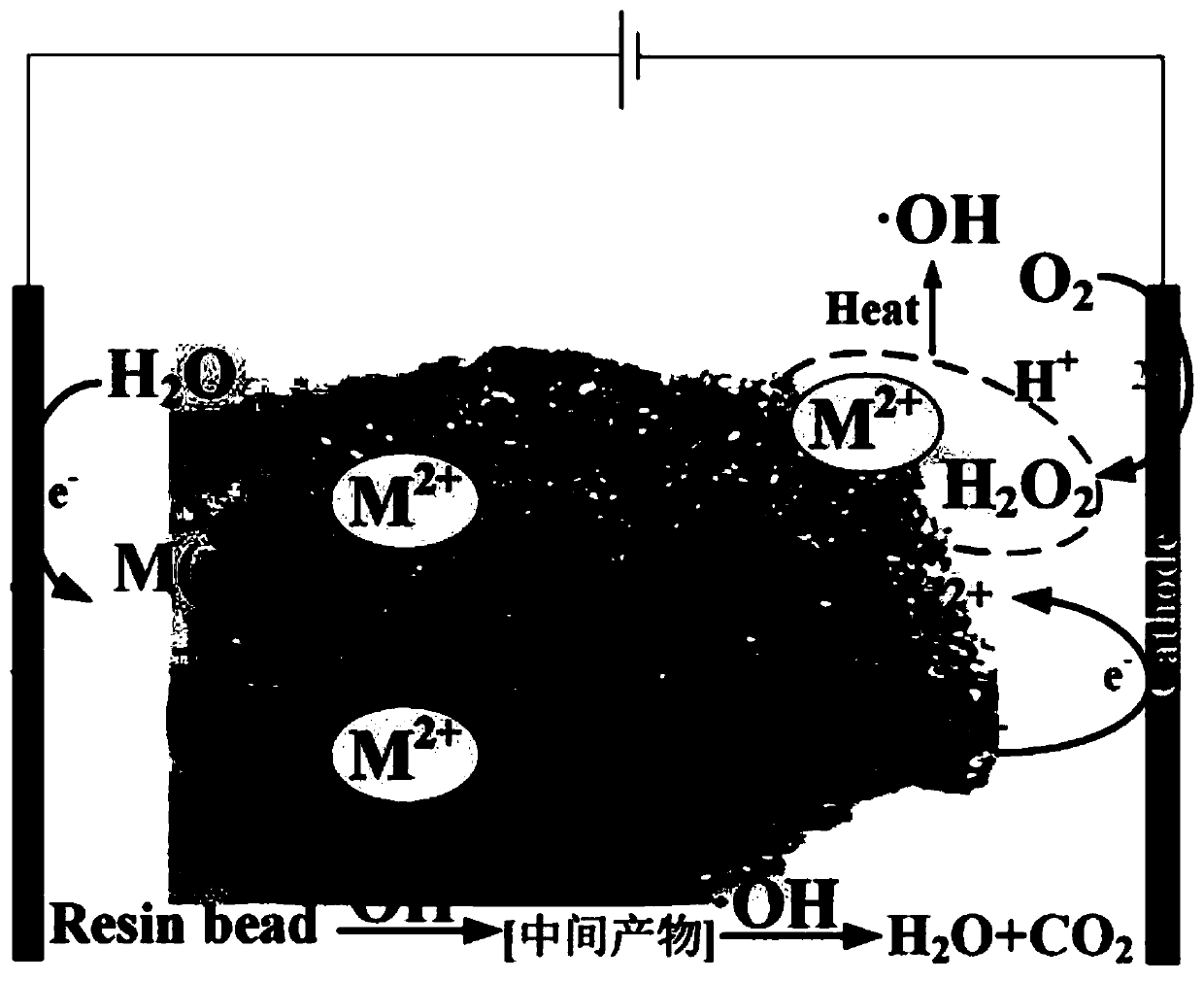
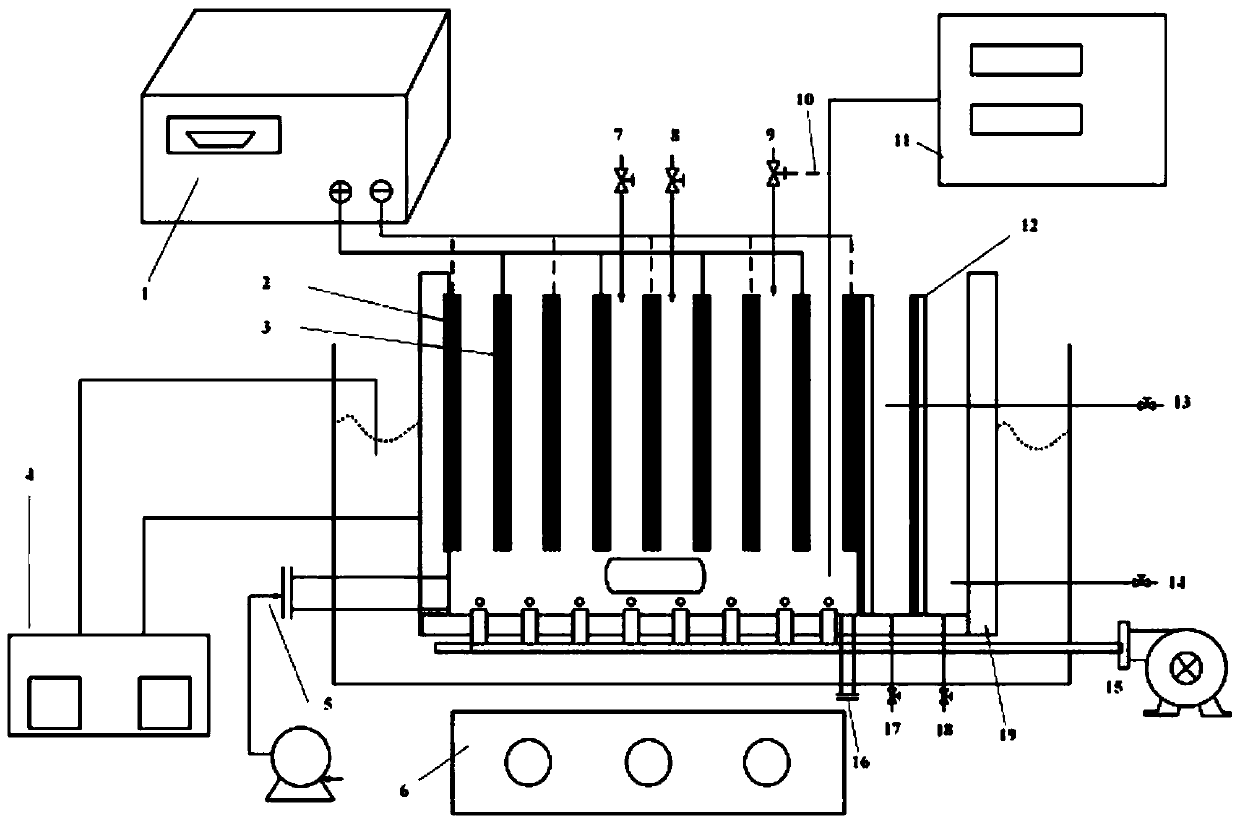
Fenton-like oxidation device for waste resin and oxidation method of device
An oxidation device and waste resin technology, applied in chemical instruments and methods, water treatment of special compounds, water/sludge/sewage treatment, etc., to achieve the effects of simple process flow, low treatment cost and high degradation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The treated waste resin is an ion exchange resin with a water content of 40wt%-60wt%.
[0037] This invention handles the device of waste resin class Fenton oxidation method such as figure 2 As shown, the device includes a reactor shell 19, a cathode plate 2, an anode plate 3, an aeration device 15, a constant current power supply device 1, a stirring device 6, a reaction rate testing device 11, a filter module 12, a temperature indicator and a control Device 4, water outlet one 17 and water outlet two 18, water body detection port one 13 and water body detection port two 14, waste material outlet 16, waste resin import 5, pH adjustment port 7, catalyst addition port 8 and H 2 o 2 Inlet 9, the cathode plate 2 and the anode plate 3 are alternately arranged in the reactor shell 19, and a reaction compartment is formed between the adjacent cathode plate 2 and the anode plate 3, and the cathode plate 2 and the anode plate 3 The constant current power supply device 1 is c...
Embodiment 2
[0054] Embodiment 2: adopt different catalyst
[0055] The catalyst in the above mixed system is selected as Fe 2+ and Co 2+ mixed catalyst.
[0056] The temperature and pH value of the reaction system are consistent, and the reaction is carried out in a device-like Fenton reactor to complete the degradation of the ion exchange resin. After the reaction is completed, the COD value of the reaction system is measured, and the raffinate and filter residue are solidified with cement.
[0057] It can be seen that when Mn 2+ When the catalyst exists, the reaction rate of the system is greater than that of Co 2+ , which may be due to the fact that Mn has more chemical valence states, thus favoring H 2 o2 decomposition. And Mn 3+ The standard reduction potential of Mn is higher, therefore, Mn 3+ Can accept electrons to convert to Mn 2+ Than Co 3+ faster.
Embodiment 3
[0058] Embodiment 3: the selection of reaction system pH
[0059] Through the pH adjustment port, the pH in the system is adjusted to 2 or 5 to complete the degradation of the ion exchange resin. After the reaction is completed, the COD value of the reaction system is measured, and the raffinate and filter residue are solidified with cement.
[0060] It can be seen that when the pH is controlled at 3, the COD value of the reaction system at the same time point is lower; in comparison, when the pH is controlled at 2 or 5, the COD value is higher. Therefore, it is recommended to control the pH of the reaction system to 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Moisture content | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com