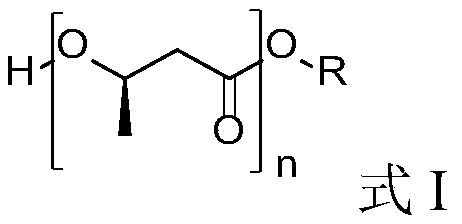
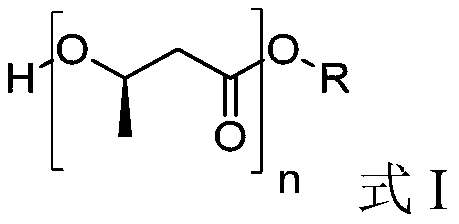
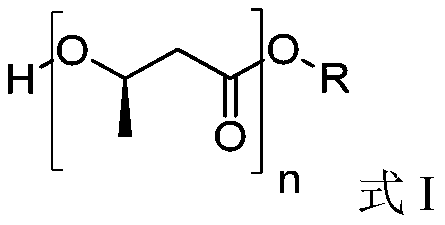
Oligomer of (R)-3-hydroxybutyric acid and preparation method of oligomer
A technology of hydroxybutyric acid and hydroxybutyrate, applied in the biological field, can solve the problems of short action time, acidosis and other problems that cannot be improved, so as to reduce the risk of acidosis and metal ion overload, long action time and prolong action time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] The preparation method of the present invention comprises the following steps:
[0047] When R is methyl, ethyl, n-propyl, isopropyl and other groups, it can be prepared by the following steps:
[0048] a) Alcoholysis: Poly(R)-3-hydroxybutyric acid alcoholysis to prepare (R)-3-hydroxybutyrate monomer
[0049] The chemical reaction equation is shown in formula II
[0050]
[0051] Poly(R)-3-hydroxybutyric acid (R)-3-hydroxybutyrate can be prepared using any alcohol in step a), such as methanol, ethanol, n-propanol, ethylene glycol, glycerol, etc., if As food, medicine or nutritional supplement, ethanol is preferred.
[0052] Suitably, the molar ratio of alcohol to poly(R)-3-hydroxybutyric acid is greater than 1:1, preferably 2:1-10:1, more preferably 3:1-6:1.
[0053] The alcoholysis in step a) is suitably carried out under the catalysis of a catalyst, which may be a Lewis acid or an organic base, preferably an inorganic acid, more preferably sulfuric acid or p-tol...
Embodiment 1
[0077] Embodiment 1 poly(R)-3-hydroxybutyric acid alcoholysis
[0078] Add 1.5L 1,2-dichloroethane and 1L absolute ethanol to a 5L three-necked flask, turn on the stirring at a speed of 150r / min, add 500g of poly(R)-3-hydroxybutyric acid after stirring for 1 minute, and stir for 3 Mix evenly for -5 minutes, then slowly add 100mL of concentrated sulfuric acid to the mixture, then slowly raise the temperature to 78°C, stop heating after 24 hours of heat preservation reaction, wait until the temperature drops below 40°C, slowly add 1L of distilled water to the reaction system, After stirring for 30 minutes, stop stirring, let stand for 30 minutes, separate layers, extract the upper aqueous phase with 500mL dichloromethane three times, combine all organic phases, wash once with 500mL saturated aqueous sodium bicarbonate solution, and then wash twice with 500mL distilled water After adding 20 g of anhydrous magnesium sulfate and drying for 2 hours, it was filtered and washed with 1...
Embodiment 2
[0079] Embodiment 2 poly(R)-3-hydroxybutyric acid alcoholysis
[0080] Add 1.5L chloroform and 1.5L methanol to a 5L three-necked flask, turn on the stirring at a speed of 150 r / min, add 500g poly(R)-3-hydroxybutyric acid after stirring for 1 minute, and stir for 3-5 minutes to mix well , then slowly add 150mL of concentrated sulfuric acid to the mixture, then slowly raise the temperature to 90°C, stop heating after 24 hours of heat preservation reaction, wait until the temperature drops below 40°C, slowly add 1L of distilled water to the reaction system, and stop after stirring for 30 minutes Stir, stand for 30 minutes, separate layers, extract the upper aqueous phase with 500mL dichloromethane three times, combine all organic phases, wash once with 500mL saturated aqueous sodium bicarbonate solution, then wash twice with 500mL distilled water, then add 20g of anhydrous sulfuric acid The magnesium was dried for 2 hours, filtered and washed with 100 mL of dichloromethane. The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com