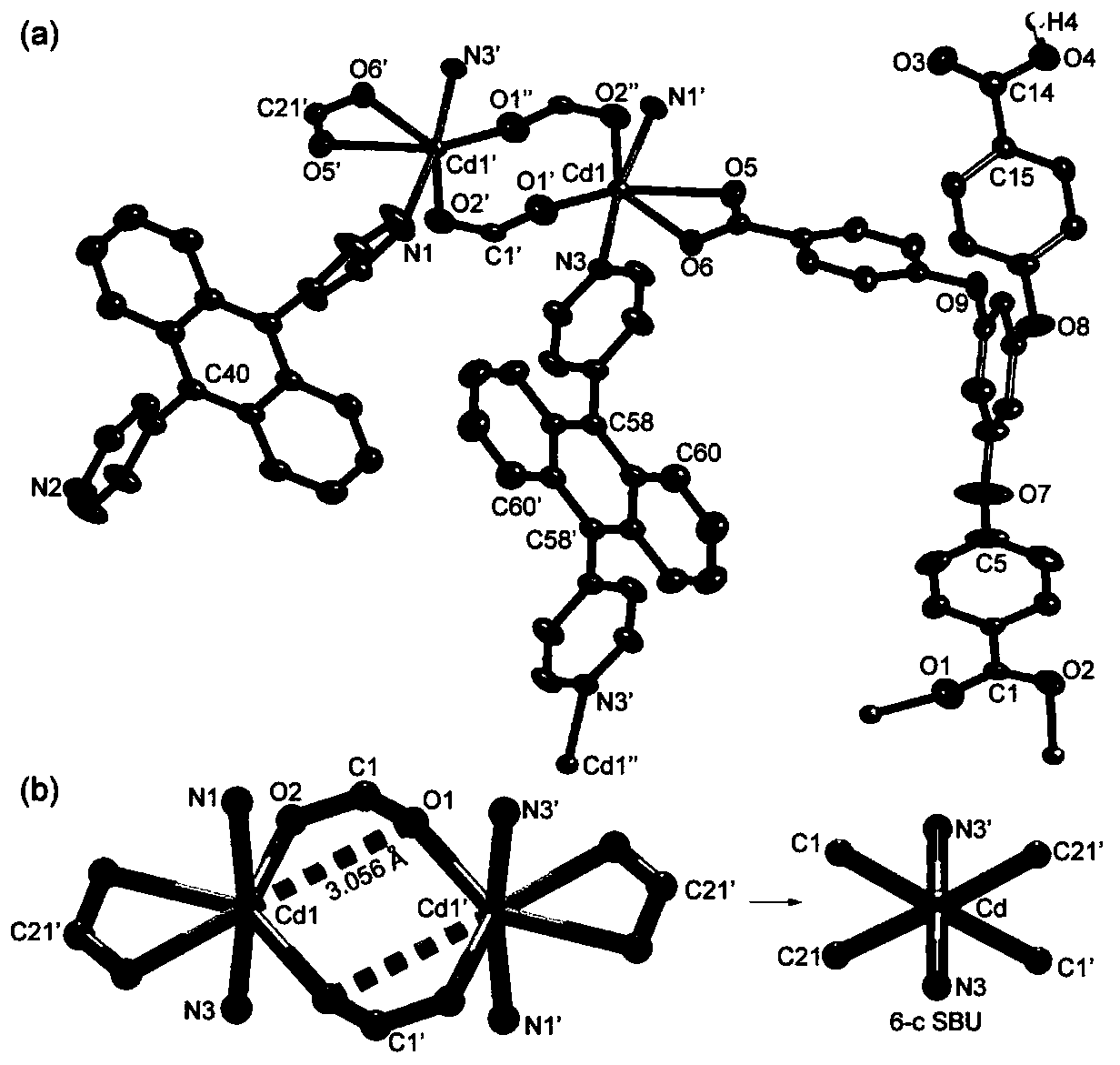
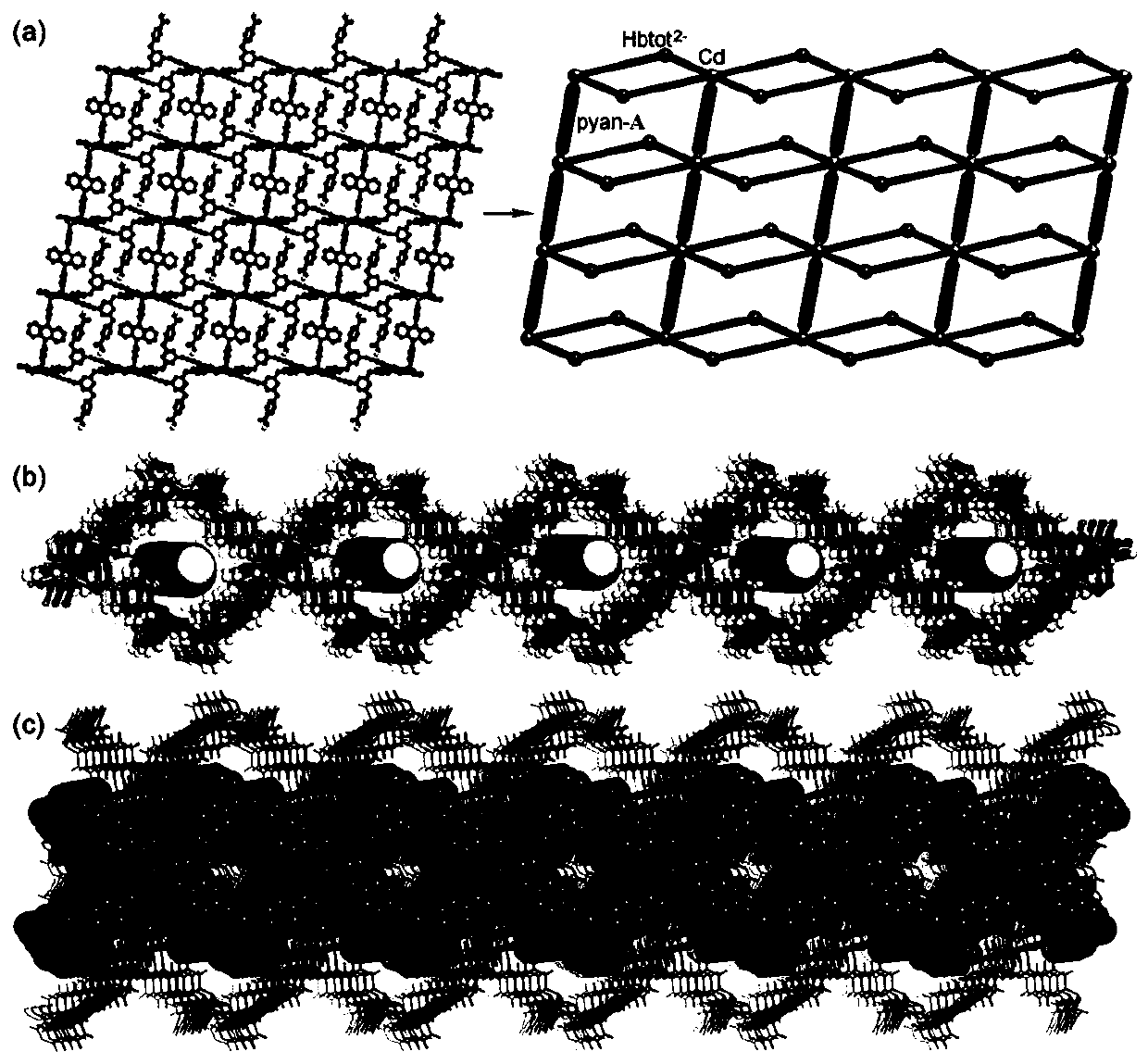
Anthryl-containing cadmium-organic supramolecular polymer, and preparation method and application thereof
A supramolecular polymer and organic technology, applied in chemical instruments and methods, analytical materials, material excitation analysis, etc., to achieve high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Take the material according to the following specific mass or volume: H 3 btot (19.4mg, 0.04mmol), pyan (13.3mg, 0.04mmol), Cd(NO 3 ) 2 4H 2 O (30.8 mg, 0.1 mmol), CH 3 CN (1mL), H 2 O (9 mL), HNO 3 (30uL, 7mol / L, 0.21mmol). Put the above materials in a 25mL reaction kettle, stir for about 10min, heat up to 160°C, react for 3 days, and cool to room temperature naturally to obtain blocky crystal samples, which are filtered from the mother liquor, washed with distilled water, and naturally cooled in the air at room temperature dry.
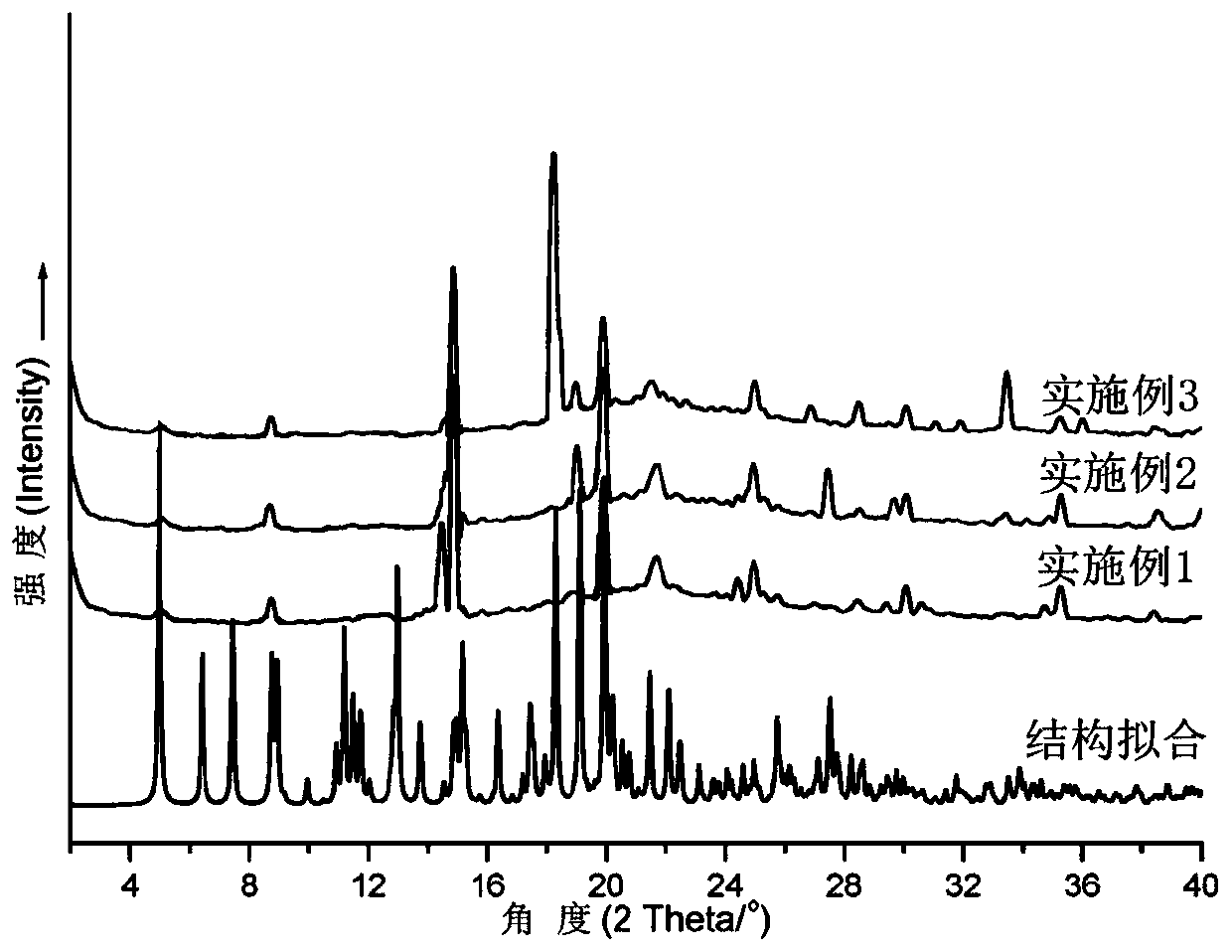
[0033] To the crystal sample of the prepared cadmium-organic supramolecular polymer containing anthracenyl, adopt Shimadzu XRD-6100 type X-ray diffractometer to carry out powder diffraction test (see image 3 , abscissa-angle; ordinate-diffraction intensity), the peak of the test spectrum and the peak of the crystal structure fitting spectrum can be well matched (software Mercury), indicating that the structure of the obtained crystal ...
Embodiment 2
[0048] Take the material according to the following specific mass or volume: H 3 btot (19.4mg, 0.04mmol), pyan (13.3mg, 0.04mmol), Cd(NO 3 ) 2 4H 2 O (18.6 mg, 0.06 mmol), CH 3 CN (1mL), H 2 O (9 mL), HNO 3 (20uL, 7mol / L, 0.14mmol). The above materials were placed in a 25mL reactor, stirred for 30min, heated to 140°C, reacted for 4 days, cooled to room temperature naturally to obtain massive crystals, which were filtered out from the mother liquor, washed with distilled water, and dried naturally in air at room temperature.
[0049] Product powder X-ray diffraction characterization (see figure 2 ), the data obtained are similar to those in Example 1. It shows that the crystal structure prepared by Example 2 does not change, and the product has high purity.
[0050] This example was repeated several times, and the mass of the supramolecular polymer actually obtained remained at 15.1-18.2 mg, and the yield calculated based on the amount of pyan was 51.7%-62.3%.
Embodiment 3
[0052] Take the material according to the following specific mass or volume: H 3 btot (19.5mg, 0.04mmol), pyan (13.3mg, 0.04mmol), Cd(NO 3 ) 2 4H 2 O (30.8 mg, 0.1 mmol), CH 3 CN (1mL), H 2 O (9 mL), HNO 3 (50uL, 7mol / L, 0.35mmol). Put the above materials in a 25mL reaction kettle, stir for about 20min, heat up to 150°C, react for 5 days, cool to room temperature naturally to obtain blocky crystals, filter them out from the mother liquor, wash with distilled water, and dry naturally in the air at room temperature .
[0053] Product powder X-ray diffraction characterization (see figure 2 ), the data obtained are similar to those in Example 1. for illustration
[0054] The crystal structure obtained in Example 3 remained unchanged and the product was relatively pure.
[0055] This example was repeated several times, and the mass of the supramolecular polymer actually obtained remained at 17.6-19.4 mg, and the yield calculated based on the amount of pyan was 60.2%-66.4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com