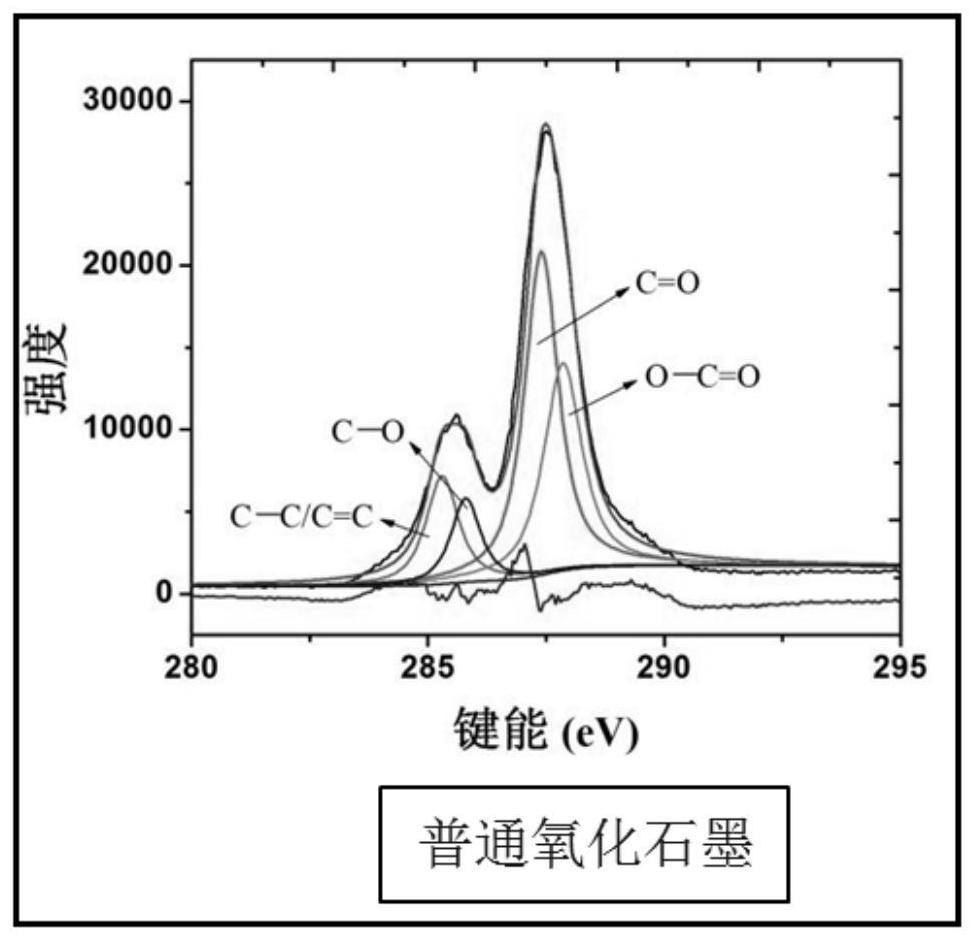
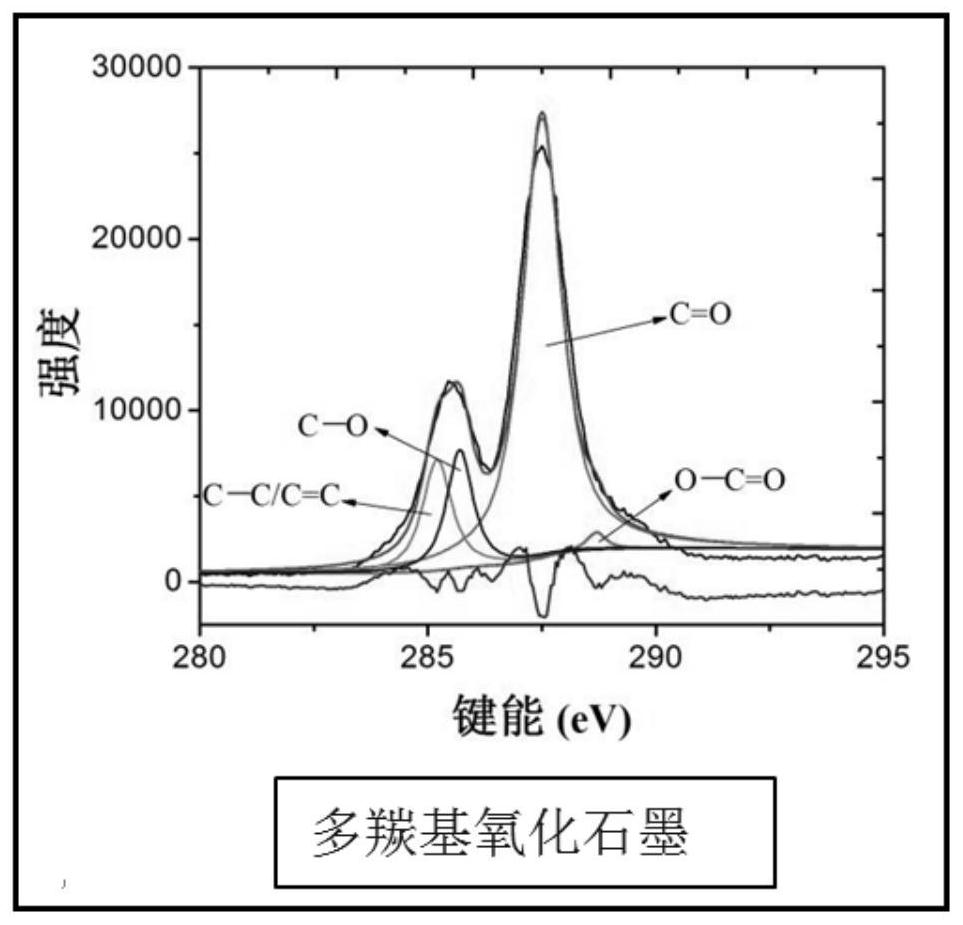
A kind of graphite oxide with multiple carbonyl functional groups and preparation method thereof
A polycarbonyl functional and carbonyl functional technology, which is applied in the field of graphite oxide with polycarbonyl functional groups and its preparation, can solve the problems of weak stability of hydroxyl and epoxy groups, inability to meet environmental protection, and endanger the personal safety of operators. The process is simple and easy, easy to modify and functionalize, and the effect of reasonable raw material preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The preparation process of the graphite oxide of the present embodiment polycarbonyl functional group is:
[0029] In a 1000mL beaker, add 180mL of 99% low-concentration fuming sulfuric acid, cool down to below 2°C, add 5g of graphite powder, stir for 1h to obtain sulfuric acid intercalated graphite, then cool down to below 2°C, slowly add potassium permanganate 20g, keep at 15±2℃, react for 48h, fully oxidize graphite.
[0030] After the reaction is over, lower the temperature to below 5°C, slowly add 180g of deionized water dropwise, control the temperature below 20°C, stir fully, keep the reaction at 5-20°C for 2 hours, control the temperature at 5-15°C and add 30% hydrogen peroxide (mass percent concentration) 20mL to obtain a soil-gray suspension, which was heated to 30°C for 1 h.
[0031] The suspension is washed with 5% (mass percent concentration) dilute hydrochloric acid by static layering, and then the graphite oxide is washed with deionized water by centrifu...
Embodiment 2
[0034] The preparation process of the graphite oxide of the present embodiment polycarbonyl functional group is:
[0035] In a 50L jacketed glass reactor, add 2400mL of 100% low-concentration fuming sulfuric acid, cool down to below 2°C, add 60g of graphite powder, stir for 2h to obtain sulfuric acid intercalated graphite, then cool down to below 2°C, and slowly add Potassium permanganate 300g, keep at 20±2℃, react for 96h, fully oxidize graphite.
[0036] After the reaction is over, lower the temperature to below 5°C, slowly add 2400g of deionized water dropwise, control the temperature below 20°C, stir well, and when the dropwise addition is complete, keep it at 5-20°C for 3 hours, control the temperature at 5-15°C and add 300mL of 30% hydrogen peroxide, A muddy gray suspension was obtained, and the temperature was raised to 30° C. for 2 h.
[0037] The suspension is washed with 5% dilute hydrochloric acid by static layering, then the graphite oxide is washed with deionized...
Embodiment 3
[0039] The preparation process of the graphite oxide of the present embodiment polycarbonyl functional group is:
[0040] In a 1000mL beaker, add 180mL of 99.9% low-concentration fuming sulfuric acid, cool down to below 2°C, add 6g of graphite powder, stir for 1.5h to obtain sulfuric acid intercalated graphite, then cool down to below 2°C, slowly add permanganate Potassium 24g, keep 20~23℃, react for 58h, fully oxidize graphite.
[0041]After the reaction is over, lower the temperature to below 5°C, slowly add 180g of deionized water dropwise, control the temperature below 20°C, stir well, and after the dropwise addition is completed, keep it at 5-20°C for 4 hours, control the temperature at 5-15°C and add 19.2mL of 30% hydrogen peroxide , to obtain a muddy gray suspension, which was heated to 35° C. for 0.5 h.
[0042] The suspension is washed with 5% dilute hydrochloric acid by static layering, then the graphite oxide is washed with deionized water by centrifugal separation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com