Preparation method of rocuronium bromide intermediate and rocuronium bromide
A technology for rocuronium bromide and intermediates, which is applied in the field of preparation of rocuronium bromide intermediates and rocuronium bromide, and can solve problems such as troublesome handling operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
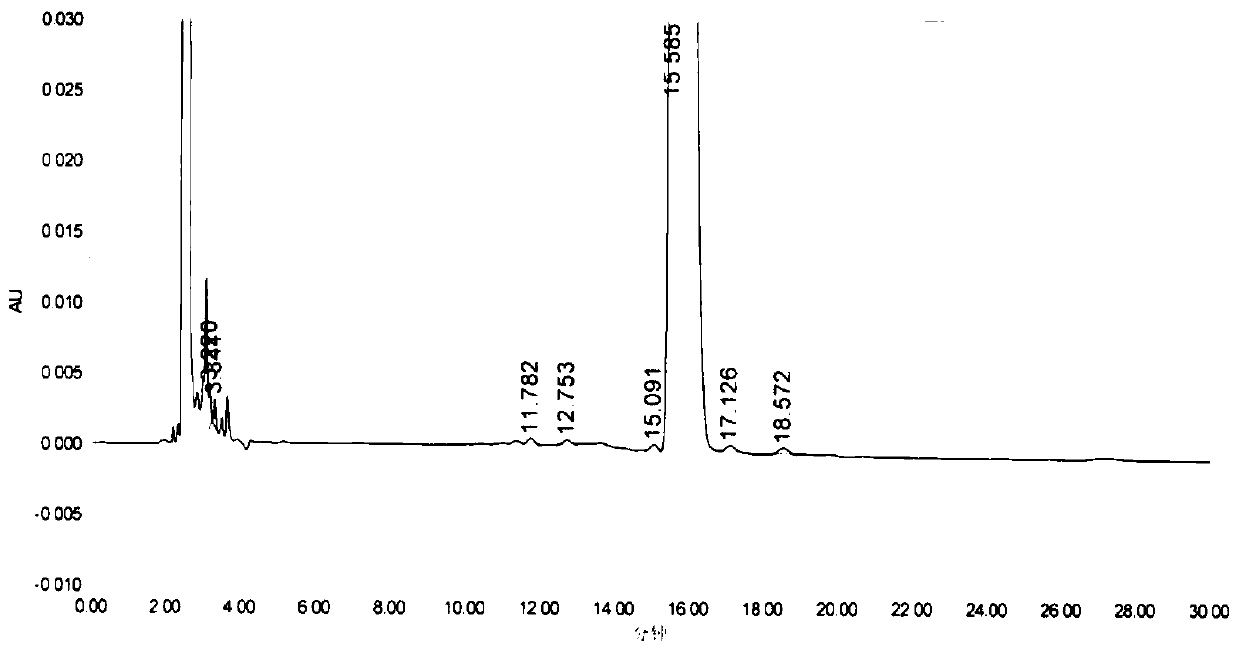
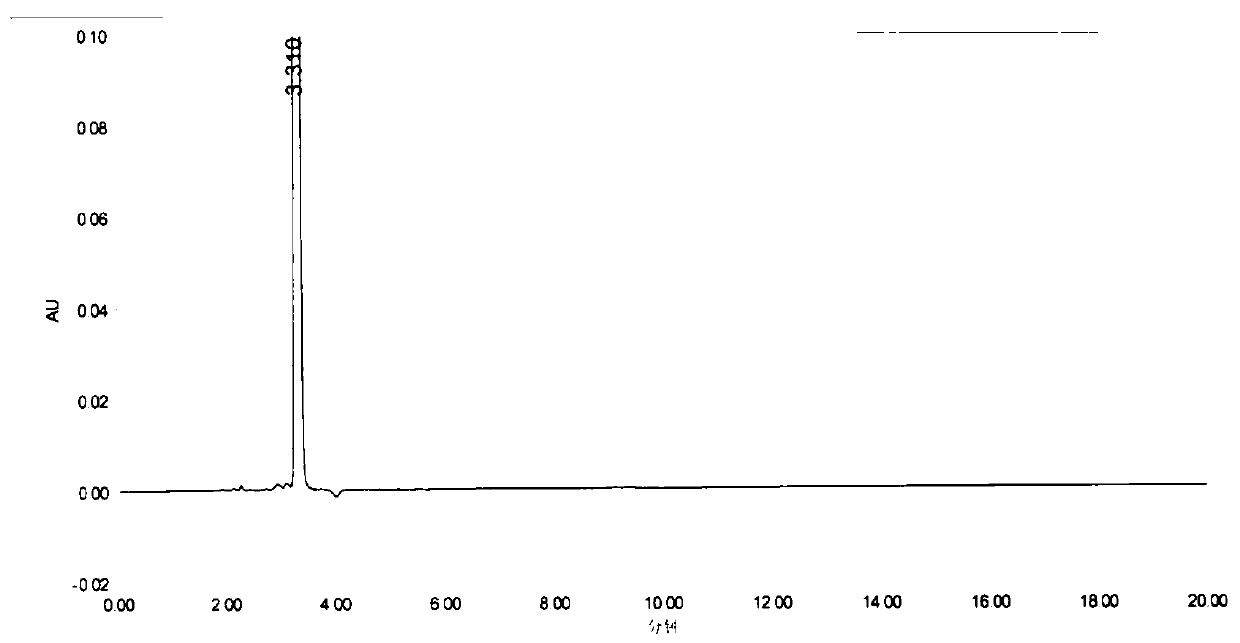
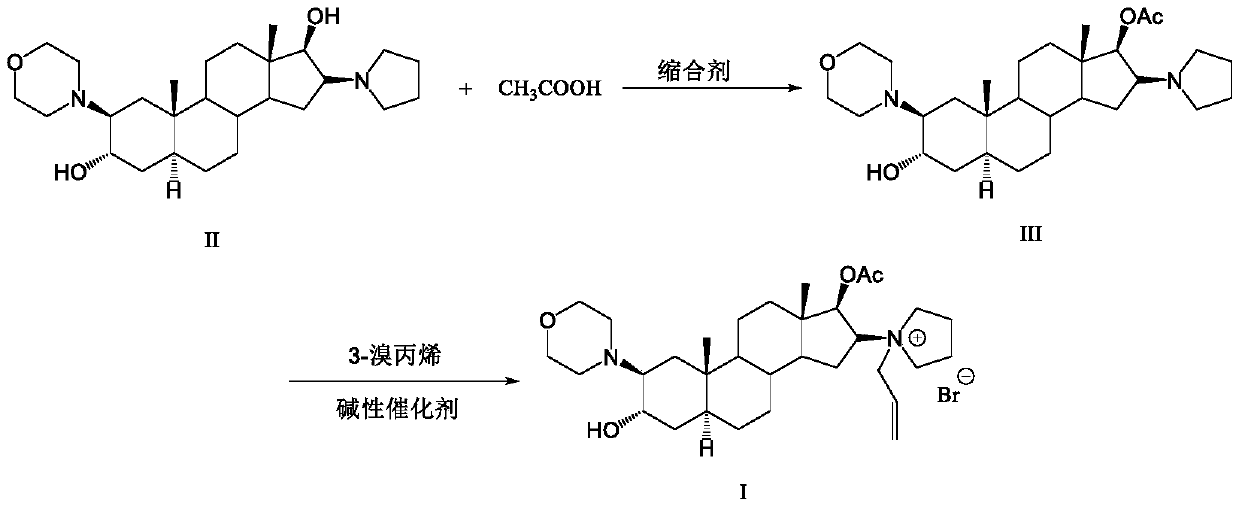
[0042] Into a dry and clean 250ml three-neck flask, add 10g of the compound of formula II, add 150ml of toluene, 30.6IIDQ, 5.4g of glacial acetic acid, and heat up to reflux for 5 hours. The temperature was lowered to 10-20°C, crystals were precipitated, filtered, and dried to obtain 7.1 g of compound (Formula III), with a molar yield of 64.9% and a purity of >99.9%.
[0043] Into a dry and clean 100ml three-neck flask, add 7g of compound of formula III, 50ml of absolute ethanol, 0.5g of sodium carbonate, 7g of 3-bromopropene, react at 35°C for 4 hours, filter under reduced pressure to remove sodium carbonate, and slowly drop the filtrate to 700ml Stir and crystallize in methyl tert-butyl ether, filter under nitrogen protection, and dry under reduced pressure at 40°C overnight to obtain 7 g of rocuronium bromide (Formula I), with a molar yield of 80.1% and a purity of >99.0%.
Embodiment 2
[0045] To a dry and clean 250ml three-neck flask, add 10g of the compound of formula II (2β, 3α, 5α, 16β, 17β)-2-(4-morpholine)-16-(1-pyrrole)-androster-3,17 -diol, add 150ml toluene, 30.6gIIDQ, 5.4g glacial acetic acid, heat up to reflux reaction for 5 hours. Cool down to 10-20°C, precipitate crystals, filter, and dry to obtain 6.8 g of compound (Formula III), with a molar yield of 62.2% and a purity of >99.9%;
[0046]To a dry and clean 100ml three-neck flask, add 6g of the compound of formula III (2β, 3α, 5α, 16β, 17β)-2-(4-morpholine)-16-(1-pyrrole)-androsta-3,17 -diol-17-acetate, 50ml absolute ethanol, 0.5g sodium carbonate, 6g 3-bromopropene, react at 35°C for 4 hours, filter under reduced pressure to remove sodium carbonate, and slowly drop the filtrate into 700ml methyl tert-butyl ether , stirred and crystallized, filtered under nitrogen protection, and dried under reduced pressure at 40°C overnight to obtain 5.9 g of rocuronium bromide (Formula I), with a molar yield...
Embodiment 3
[0048] Into a dry and clean 2000ml three-neck flask, add 50g of the compound of formula II, add 0.5L of acetone, 124.6g of EEDQ, and 26.9g of glacial acetic acid, raise the temperature to reflux for 4 hours, and then add 0.75L of acetonitrile. Then slowly lower the temperature to room temperature, heat and crystallize for 2 hours, and filter to obtain 48 g of white crystalline powder (Formula III), with a molar yield of 87.7% and a purity of >99.9%;
[0049] Into a dry and clean 1000ml three-necked flask, add 45g of the compound of formula III, 900ml of tert-butanol, 10g of alumina, 50g of 3-bromopropene, react at 45°C for 6 hours, filter under reduced pressure to remove the alumina, and slowly drop the filtrate to 5L without Stir and crystallize in water ether, filter under nitrogen protection, and dry under reduced pressure at 40°C overnight to obtain 50 g of rocuronium bromide (Formula I), with a molar yield of 89.3% and a purity of >99.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com