Modified epsilon-polylysine and preparation method thereof, and application of modified epsilon-polylysine in turbot preservation
A technology of polylysine and turbot, which is applied in the direction of chemical preservation of meat/fish, food ingredients as antimicrobial preservation, food ingredients containing organic compounds, etc., can solve unsatisfactory, short antibacterial time, freshness In the later stage, the ideal effect cannot be achieved, and the effect of prolonging the shelf life, simple preparation method, and mild reaction conditions is achieved.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The synthetic route of modified ε-polylysine is as follows:
[0040]
[0041] The specific synthetic steps of modified ε-polylysine are as follows:
[0042] to N α - tert-butoxycarbonyl-L-lysine (0.24g, 1mmol), tert-butylisonitrile (0.18g, 2mmol) was added to the MeOH suspension of benzaldehyde (0.23 g, 2mmol), and the resulting mixture was stirred at room temperature for 48 After 2 hours, the methanol was spun off on a rotary evaporator, and the crude product was dissolved in CH 2 Cl 2 , adding petroleum ether for multiple precipitations, filtering, and drying the obtained solid under vacuum conditions to obtain a white solid polymer 1.
[0043] To polymer one (1.0 g) anhydrous CH at 0 °C 2 Cl 2 Trifluoroacetic acid (1 mL) was slowly added to the solution, the mixture was stirred at room temperature for 3 h, and the CH 2 Cl 2 , and then add petroleum ether to precipitate several times, filter and dry to obtain a white solid which is modified ε-polylysine.
...
Embodiment 2
[0048] The specific synthetic steps of modified ε-polylysine are as follows:
[0049] to N α - tert-butoxycarbonyl-L-lysine (0.24g, 1mmol), tert-butylisonitrile (0.27g, 3mmol) was added to the MeOH suspension of benzaldehyde (0.345 g, 3mmol), and the resulting mixture was stirred at room temperature for 72 After 2 hours, the methanol was spun off on a rotary evaporator, and the crude product was dissolved in CH 2 Cl 2 , adding petroleum ether for multiple precipitations, filtering, and drying the obtained solid under vacuum to obtain a white solid polymer 1.
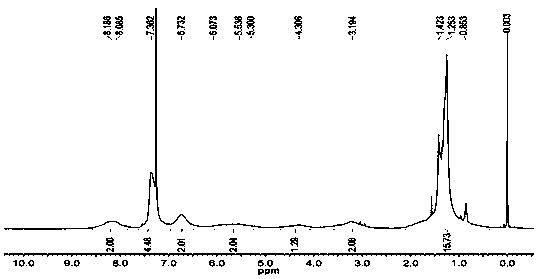
[0050] To polymer one (1.0 g) anhydrous CH at 0 °C 2 Cl 2 Trifluoroacetic acid (1.1 mL) was slowly added to the solution, the mixture was stirred at room temperature for 6 hours, and the CH 2 Cl 2 , and then add petroleum ether to precipitate several times, filter and dry to obtain a white solid which is modified ε-polylysine. The present embodiment modified ε-polylysine 1 H NMR spectrum as figure 1 , see the in...
Embodiment 3
[0053] The specific synthetic steps of modified ε-polylysine are as follows:
[0054] to N α - tert-butoxycarbonyl-L-lysine (0.24g, 1mmol), tert-butylisonitrile (0.36g, 4mmol) was added to the MeOH suspension of benzaldehyde (0.46 g, 4mmol), and the resulting mixture was stirred at room temperature for 96 After 2 hours, the methanol was spun off on a rotary evaporator, and the crude product was dissolved in CH 2 Cl 2 , adding petroleum ether for multiple precipitations, filtering, and drying the obtained solid under vacuum to obtain a white solid polymer 1.
[0055] To polymer one (1.0 g) anhydrous CH at 0 °C 2 Cl 2 Trifluoroacetic acid (1.2 mL) was slowly added to the solution, the mixture was stirred at room temperature for 8 hours, and the CH 2 Cl 2 , and then add petroleum ether to precipitate several times, filter and dry to obtain a white solid which is modified ε-polylysine. The present embodiment modified ε-polylysine 1 H NMR spectrum as figure 1 , see the inf...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com