Method for preparing high-purity (R)-4-formyl-2,2-dimethyl-3-oxazoline tert-butyl carboxylate
An oxazoline carboxylic acid, high-purity technology, applied in organic chemistry methods, organic chemistry, etc., can solve the problems of high risk factor, unfavorable industrial production, and high cost, and achieve the effects of less three wastes, favorable production operation, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
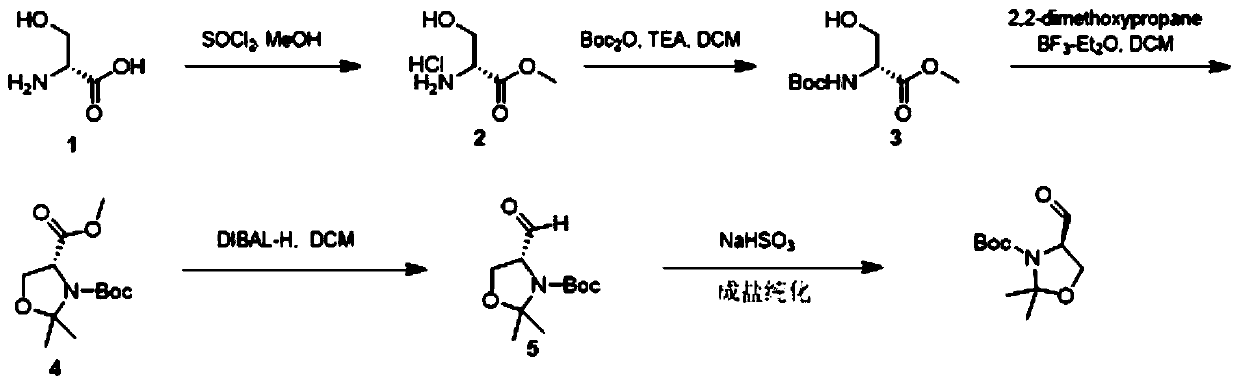
[0053] like figure 1Shown, the present invention prepares the method for high-purity (R)-4-formyl-2,2-dimethyl-3-oxazoline carboxylic acid tert-butyl ester, comprises the following steps:
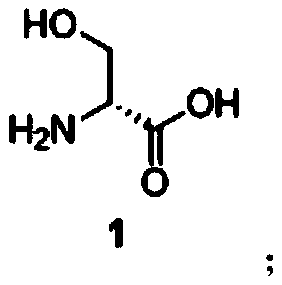
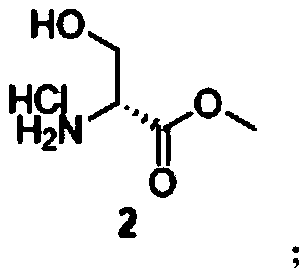
[0054] S1. Preparation of compound 2 from compound 1: reacting compound 1 with thionyl chloride in methanol to obtain compound 2;
[0055] S2. Preparation of compound 3 from compound 2: reacting compound 2 with Boc anhydride under triethylamine to obtain compound 3;
[0056] S3. Compound 4 was prepared from compound 3: compound 4 was obtained by reacting compound 3 with 2,2-dimethoxypropane under boron trifluoride diethyl ether;
[0057] S4. Compound 5 was prepared from compound 4: compound 4 was reduced with DIBAL-H at -60 to -80°C to obtain a crude product, and then reacted with sodium sulfite to form a salt for purification to obtain high-purity (R)-4-formyl-2 , tert-butyl 2-dimethyl-3-oxazolinecarboxylate.
[0058] The synthetic route is as follows:
[0059]
[0060] The present ...
Embodiment 2
[0062] Based on Example 1, the present invention prepares the method for high-purity (R)-4-formyl-2,2-dimethyl-3-oxazoline carboxylic acid tert-butyl ester, the specific steps are as follows:
[0063] S1, compound 2 is prepared from compound 1, the synthetic route is as follows:
[0064]
[0065] Add methanol (4.2L) and compound 1 (0.7kg, 6.66mol) into a 5L clean and dry four-neck flask at room temperature, under nitrogen protection, cool down to 0-5°C in an ice-salt water bath while stirring; maintain the system temperature at 0-5°C , dropwise added thionyl chloride (0.9075kg, 7.63 mol, took 6h), the system was white and turbid; after the dropwise addition, the system was slowly warmed to room temperature for 15 hours.
[0066] The reaction solution was transferred to a 5L clean and dry one-necked bottle and concentrated under reduced pressure until no obvious fraction flowed out to obtain compound 2 (1.036kg, 6.66mol, crude product yield=100%).
[0067] S2, prepare compo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com