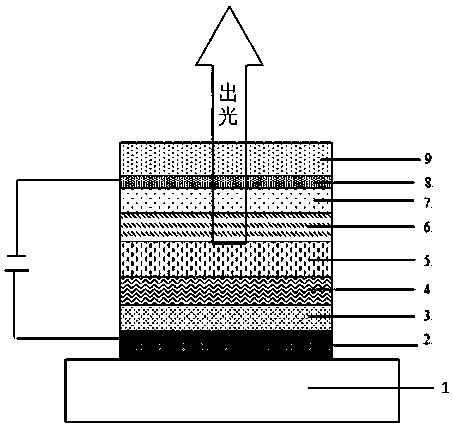
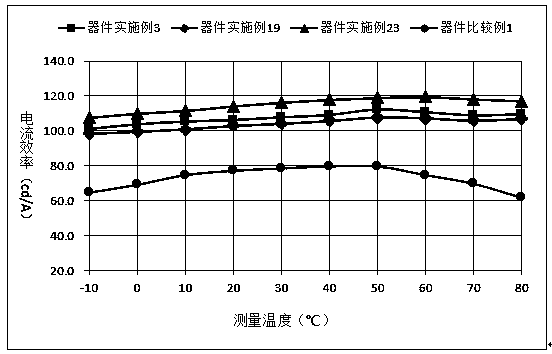
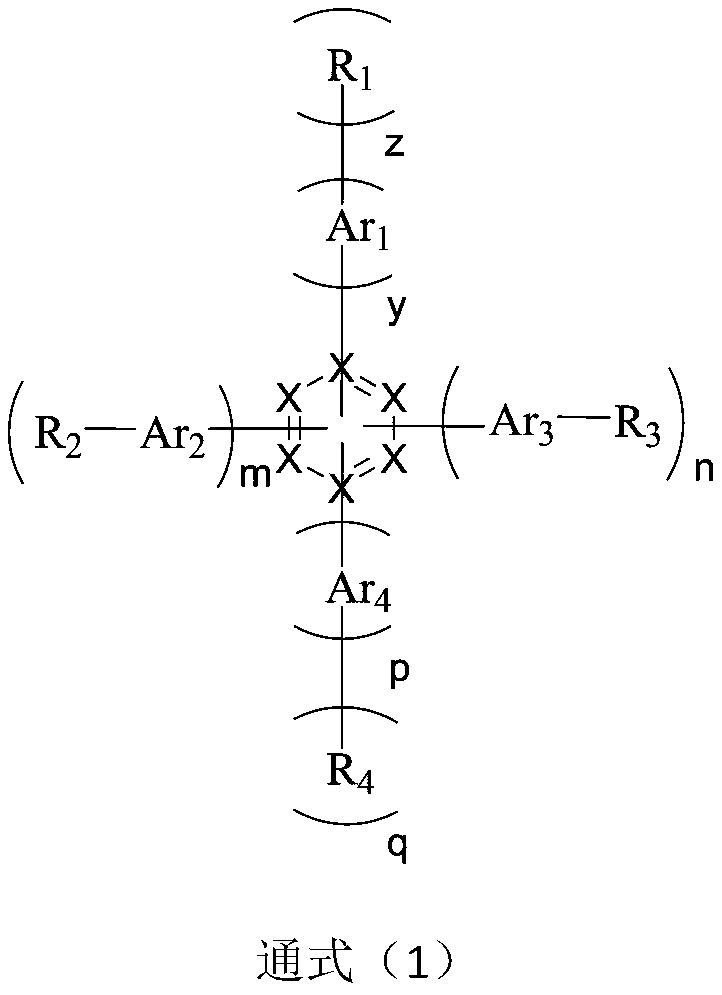
Organic compound based on azabenzene and anthrone structures, and applications in OLED devices
An organic compound, azabenzene technology, applied in silicon organic compounds, compounds of group 4/14 elements of the periodic table, organic chemistry, etc., can solve problems affecting the angular distribution of OLED radiation spectrum and complex manufacturing process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0198] Embodiment 1: the synthesis of intermediate
[0199] 1) Synthesis of Intermediate A
[0200] a) When Ar1 、Ar 4 not represented as a single bond, or Ar 1 、Ar 4 is a single bond, and R 1 , R 4 When linked to the mother core azabenzene C-C:
[0201]
[0202] Under a nitrogen atmosphere, the raw material I was weighed and dissolved in tetrahydrofuran (THF), and then bis(pinacolyl)diboron, (1,1'-bis(diphenylphosphino)ferrocene)dichloropalladium ( II) and potassium acetate, stirring the mixture, reacting the mixed solution of the above reactants at 60-100°C for 5 hours; after the reaction, cooling and adding water to the obtained reaction mixture to precipitate the reaction product, filtering the mixture, and taking The filter cake was dried in a vacuum oven, and the obtained residue was separated and purified through a silica gel column, and the eluent used was dichloromethane:petroleum ether=0.1~3:1 to obtain intermediate E-a;
[0203] The molar ratio of the raw m...
Embodiment 2
[0258] Embodiment 2: the synthesis of compound 1:
[0259]
[0260] In a 250mL three-neck flask, nitrogen gas was introduced, and 0.01mol of raw material A1, 150ml of DMF, 0.03mol of intermediate B1, 2.0×10 -4 mol palladium acetate, stir, then add 0.03mol 1mol / L of K 3 PO 4 Aqueous solution, heated to 150°C, refluxed for 24 hours, sampled and spotted on plate, reacted completely, cooled naturally, extracted with 200mL dichloromethane and 200mL water, separated, dried the extract containing the reaction product with anhydrous sodium sulfate, filtered , the filtrate was rotary evaporated, purified by a silica gel column with dichloromethane:petroleum ether=3:1 eluent, and the target product was obtained with an HPLC purity of 99.4% and a yield of 62.8%.
[0261] Elemental analysis structure (molecular formula C 54 h 32 N 2 o 4 ): theoretical value C, 83.92; H, 4.17; N, 3.62; 0, 8.28; test value: C, 83.93; ESI-MS(m / z)(M + ): The theoretical value is 772.24, and the mea...
Embodiment 3
[0262] Embodiment 3: the synthesis of compound 5:
[0263]
[0264] The preparation method of compound 5 is the same as that of Example 2, except that intermediate B1 is replaced by intermediate B2.
[0265] Elemental analysis structure (molecular formula C 52 h 30 N 4 o 4 ): theoretical value C,80.61; H,3.90; N,7.23; O,8.26; test value: C,80.63; H,3.29; N,7.25; O,8.24. ESI-MS(m / z)(M + ): The theoretical value is 774.23, and the measured value is 774.35.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com