Coated catalyst, preparation method thereof and application of coated catalyst in fuel cell
A fuel cell and cladding technology, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of high production cost, difficult to store, easy to cause cancer, etc., achieve good corrosion resistance, good catalytic effect, and increase active sites point effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
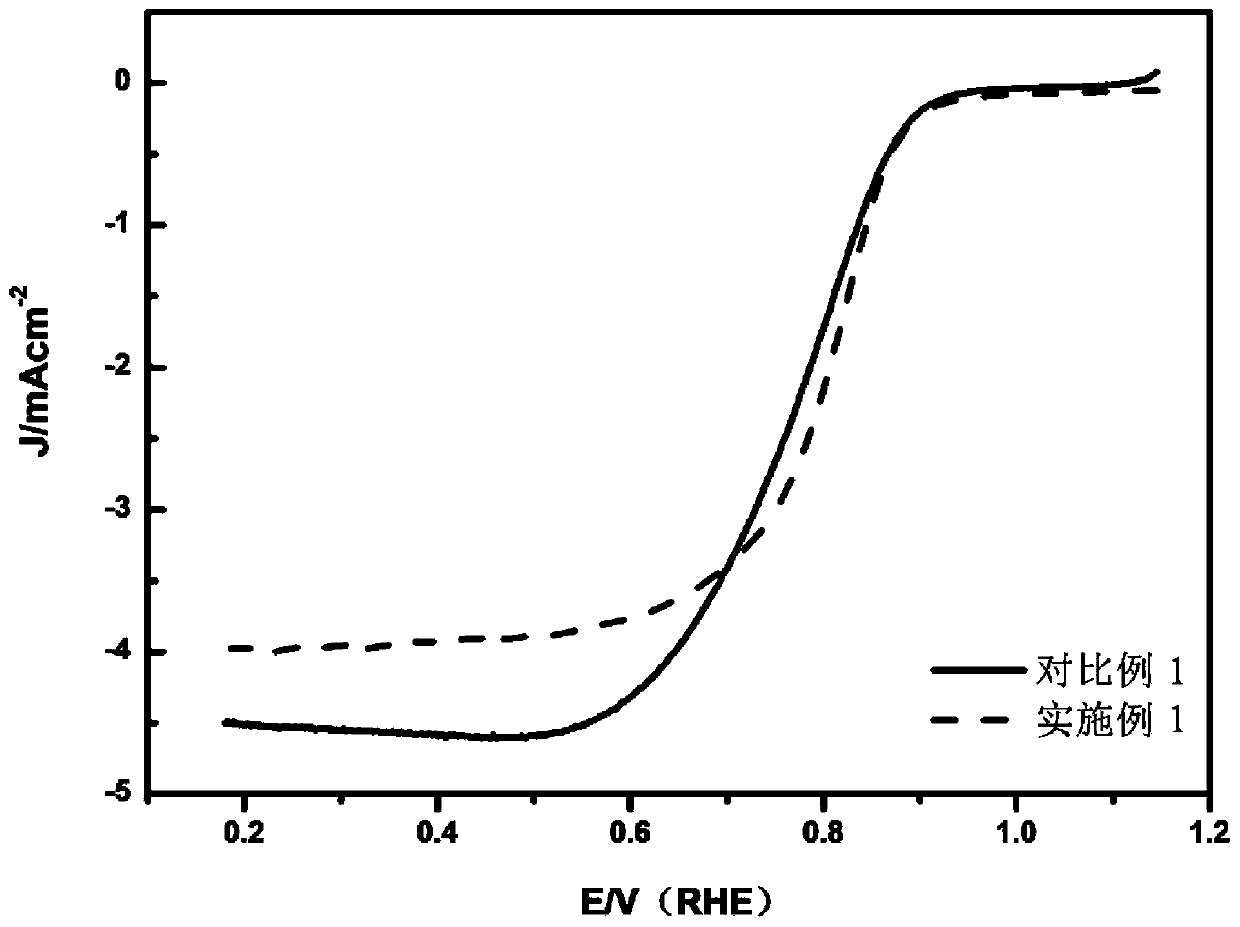
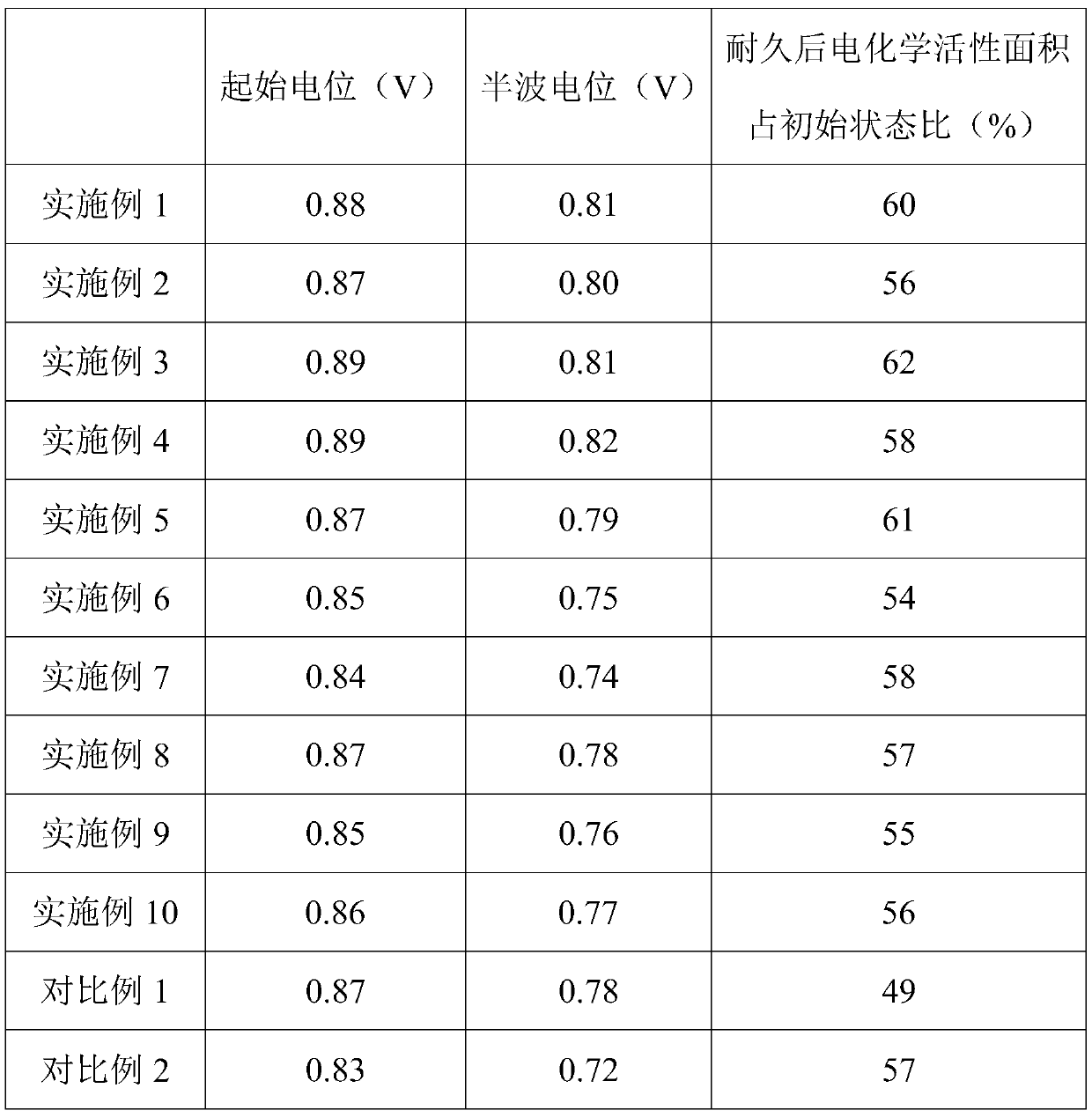
Embodiment 1
[0075] (1) Put 1.2g of ferrous chloride and 3.2g of ferric chloride into a 100mL beaker, add them to 40mL of deionized solution, pass through nitrogen protection, and drop into 20mL of a solution containing 4g of NaOH while stirring , stir for 30min;
[0076] (2) The above solution was transposed into a polytetrafluoroethylene-lined stainless steel reactor for hydrothermal carbonization reaction, and the temperature was raised to 160° C. for 24 hours;
[0077] (3) After the liquid is naturally cooled, it is separated by suction filtration, and the solid is repeatedly washed with deionized water and ethanol until the filtrate is clear;
[0078] (4) After drying the washed solid in a vacuum oven at 70°C for 10 hours, place it in a tube furnace and roast it at 400°C for 3 hours to obtain ferric oxide nanoparticles;
[0079] (5) Add ferric iron tetroxide prepared in step (4) into 120mL Tris-HCl pH=8.5 buffer solution, take 2.8g dopamine hydrochloride, ultrasonicate for 30min to o...
Embodiment 2
[0084] (1) Put 1.3g of cobalt chloride into a 100mL beaker, add 40mL of deionized solution to make a solution, drop in 20mL of a solution containing 1.4g of KOH while stirring, and stir for 10min;
[0085] (2) The above solution was transposed into a polytetrafluoroethylene-lined stainless steel reactor for hydrothermal carbonization reaction, and the temperature was raised to 170° C. for 18 hours;
[0086] (3) After the liquid is naturally cooled, it is separated by suction filtration, and the solid is repeatedly washed with deionized water and ethanol until the filtrate is clear;
[0087] (4) After drying the washed solid in a vacuum oven at 90° C. for 8 hours, place it in a tube furnace and roast it at 500° C. for 2 hours to obtain cobalt trioxide nanoparticles;
[0088] (5) Add the cobalt trioxide nanoparticles prepared in step (4) into 100mL Tris-HCl pH=8.5 buffer solution, take 1.9g of dopamine hydrochloride, ultrasonicate for 20min to obtain a uniform dispersion, stir f...
Embodiment 3
[0093] (1) Put 1.8g of nickel nitrate into a 100mL beaker, add it to 40mL of deionized solution, add 0.5g of NH 3 Ammonia solution 10mL, stirred for 20min;
[0094] (2) The above solution was transposed into a polytetrafluoroethylene-lined stainless steel reactor for hydrothermal carbonization reaction, and the temperature was raised to 200° C. for 12 hours;
[0095] (3) After the liquid is naturally cooled, it is separated by suction filtration, and the solid is repeatedly washed with deionized water and ethanol until the filtrate is clear;
[0096] (4) Place the washed solid in a vacuum drying oven at 100°C for 6 hours, then place it in a tube furnace and roast it at 600°C for 2 hours to obtain nickel oxide nanoparticles;
[0097] (5) Add the nickel oxide nanoparticles prepared in step (4) into 150mL Tris-HCl pH=8 buffer solution, take 3.7g of dopamine hydrochloride, ultrasonicate for 40min to obtain a uniform dispersion, stir for 24h, filter with deionized After washing w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com