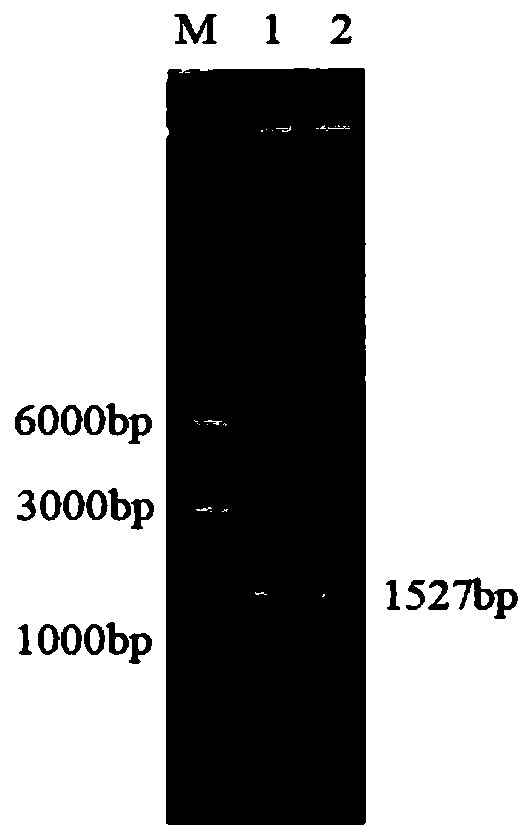
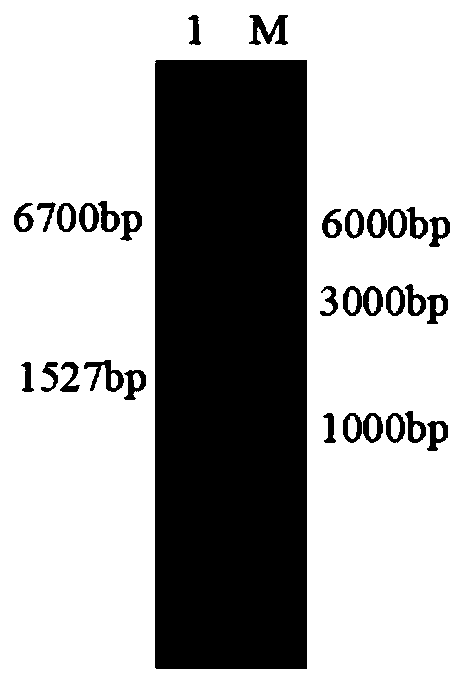
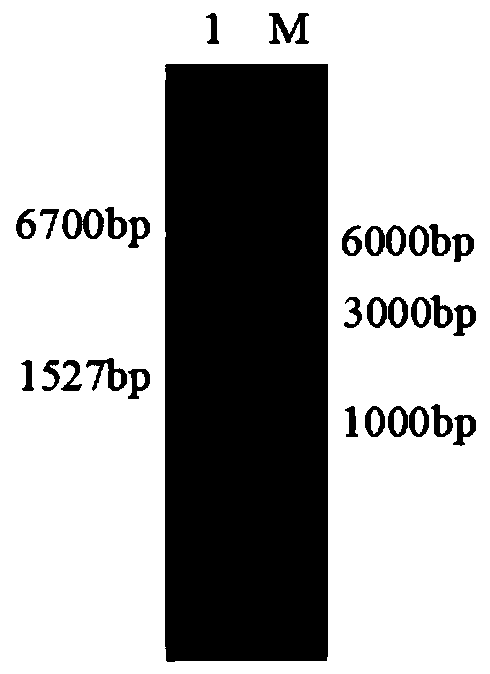
Novel phospholipase D and method for preparing functional phospholipids by using same
A phospholipase and mutant technology, applied in the direction of microorganism-based methods, botany equipment and methods, biochemical equipment and methods, etc., can solve the problems of long fermentation cycle and complicated culture conditions of Streptomyces
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1: the acquisition of wild-type phospholipase D gene
[0063] 1. The wild-type phospholipase D gene comes from a strain of Streptomyces antibioticus, and its genomic DNA is extracted.
[0064] Wherein the extraction steps of antibiotic Streptomyces genomic DNA are as follows:
[0065] (1) Pick a ring of bacteria from the culture plate and inoculate in 50mL of appropriate medium, culture at 26°C, 150r / min for 2-3d.
[0066] (2) After that, take 1 mL of the culture solution in a 1.5 mL EP tube, centrifuge at 8000 r / min for 20 min, pour off the supernatant, and resuspend with 200 μL of solution I or sterilized water.
[0067] (3) Add 20-50μL of 50mg / mL lysozyme and digest at 37°C for 0.5-1h.
[0068] (4) Add 100 μL of 2% SDS solution and fully react until the bacterial suspension becomes viscous.
[0069] (5) Add an equal volume of Tris to balance phenol:chloroform=1:1, mix well, centrifuge at 12000r / min for 5min, and transfer the supernatant to another EP tu...
Embodiment 2
[0080] Embodiment 2: the screening of high activity phospholipase D gene
[0081] 1. Random mutation based on error-prone PCR technology to construct a new type of phospholipase D, and design primers as follows:
[0082] Upstream P1 (SEQ ID NO: 5): CCGGAATTCGGCGGACACACCGCC
[0083] Downstream P2 (SEQ ID NO: 6): AAGGAAAAAAGCGGCCGCGCCCGCCTGGCG
[0084] In the error-prone PCR reaction system, P1 and P2 were used as upstream and downstream primers, and pET22b-pld, the recombinant vector in which the wild-type phospholipase D gene was connected to the pET22b vector, was used as a template to perform error-prone PCR.
[0085] The reaction conditions for its amplification are:
[0086]
[0087]
[0088] Amplification conditions were: pre-denaturation at 95°C for 10 min; denaturation at 98°C for 10 s, annealing at 53°C for 30 s, extension at 72°C for 1 min and 45 s for 30 cycles; extension at 72°C for 10 min.
[0089] 2. Cloning the phospholipase D error-prone PCR product int...
Embodiment 3
[0102] Example 3: On the basis of a single amino acid mutation, a phospholipase D variant with multiple amino acid mutations is obtained. Taking the overlapping PCR technique as an example to carry out N153I, G270F, P316W, and A452F mutations on the basis of the pldmD84I mutant, the final gene sequence is as follows: Shown in SEQ ID NO:3, the final amino acid sequence is shown in SEQ ID NO:4.
[0103] The specific strategy is: first realize the double mutation on the basis of a single mutation, and then carry out the mutation of the third, fourth and fifth amino acids.
[0104] First realize the mutation of N153I on the basis of D84I, the steps are the same as in Example 2, and the overlapping primers are designed as follows:
[0105] Upstream P1 (SEQ ID NO.5): CCGGAATTCGGCGGACACACCGCC
[0106] Downstream P2 (SEQ ID NO. 6): AAGGAAAAAAGCGGCCGCGCCCGCCTGGCG
[0107] Overlapping primer P5 (SEQ ID NO.7): CGGCAAGGTCACGCTCATCGTCGCCTC
[0108] Overlapping primer P6 (SEQ ID NO.8): G...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com