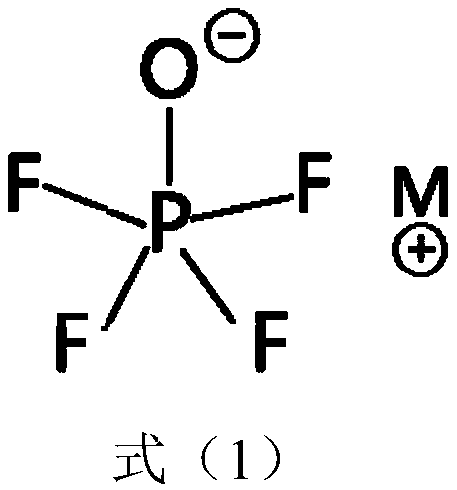
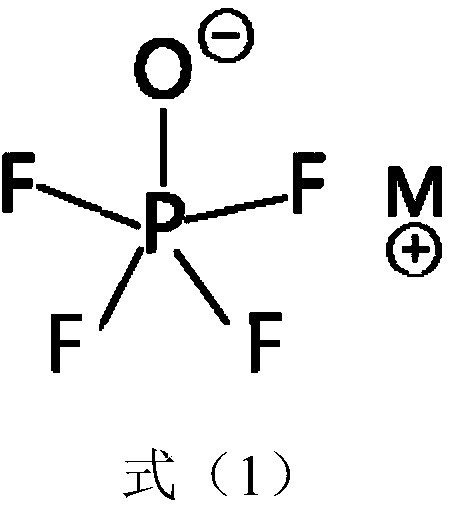
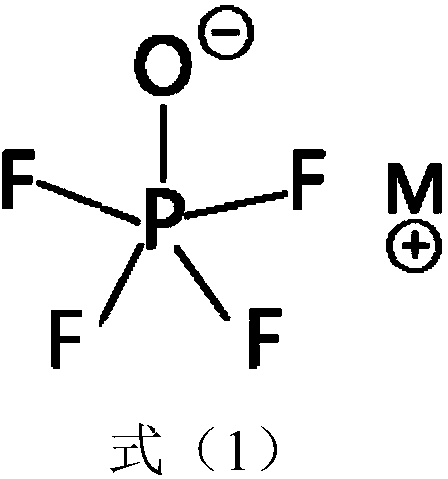
Lithium secondary battery electrolyte with low internal resistance and lithium secondary battery
A lithium secondary battery and electrolyte technology, applied in secondary batteries, secondary battery repair/maintenance, non-aqueous electrolyte storage batteries, etc., can solve the problem of unstable battery cycle, affecting the overall performance of the battery, and complex SEI film composition and morphology and other problems, to achieve the effect of reducing internal resistance and good low temperature performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Preparation of positive electrode sheet of lithium secondary battery
[0031] The positive electrode active material lithium nickel cobalt manganese oxide (LiNi 1 / 3 co 1 / 3 mn 1 / 3 o 2 ), the conductive agent Super-P, and the binder PVDF are dissolved in the solvent N-methylpyrrolidone at a mass ratio of 96:2.0:2.0 and mixed evenly to make a positive electrode slurry, and then the positive electrode slurry is evenly coated on the current collector aluminum foil , coating amount is 0.018g / cm 2 , followed by drying at 85°C, cold pressing, trimming, cutting, and stripping, and then drying at 85°C for 4 hours under vacuum conditions, welding the tabs, and making a positive electrode sheet of a lithium secondary battery that meets the requirements.
[0032] (2) Preparation of the negative electrode sheet of the lithium secondary battery
[0033] Negative electrode active material graphite, conductive agent Super-P, thickener CMC, and binder SBR are dissolved in solven...
Embodiment 2
[0039] A lithium secondary battery was prepared according to the method of Example 1, except that the electrolyte of the lithium secondary battery was lithium hexafluorophosphate accounting for 10.0% of the total mass of the electrolyte as a lithium salt, and the non-aqueous organic solvent was ethylene carbonate and ethyl methyl carbonate. Accounting for 85.5% of the total mass of the electrolyte, the mass ratio is 1:2. Add sodium tetrafluorophosphate, accounting for 0.5% of the total mass of the electrolyte. The second additive is fluoroethylene carbonate and lithium difluorophosphate, accounting for 3.0% and 1.0% of the total mass of the electrolytic solution respectively. The cathode material used in lithium secondary batteries is LiNi 0.8 co 0.1 mn 0.1 o 2 .
Embodiment 3
[0041] A lithium secondary battery was prepared according to the method of Example 1, except that the electrolyte of the lithium secondary battery was lithium hexafluorophosphate accounting for 13.0% of the total mass of the electrolyte as a lithium salt, and the non-aqueous organic solvent was ethylene carbonate and ethyl methyl carbonate. Accounting for 85.0% of the total mass of the electrolyte, the mass ratio is 1:3. Potassium tetrafluorophosphate was added, accounting for 0.5% of the total mass of the electrolyte. The second additive is fluoroethylene carbonate, accounting for 1.5% of the total mass of the electrolyte. The cathode material used in lithium secondary batteries is LiNi 0.8 co 0.15 al 0.05 o 2 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com