Metronidazole tablet and preparation method thereof
A technology of metronidazole tablets and metronidazole, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as unsatisfactory dissolution effects and poor drug efficacy, To achieve the effect of improving dissolution effect, improving quality and curative effect, and good dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060]The second aspect of the embodiments of the present invention provides a method for preparing metronidazole tablets, comprising the following steps: Step S10: pulverize the metronidazole raw material and pass through a first mesh sieve to obtain metronidazole, the metronidazole The particle size of nidazole is not greater than 300 microns.
[0061] The mesh number of the first mesh sieve can be set as required, for example, it can be 60 mesh sieves. After pulverizing the metronidazole raw material, the particle size of the metronidazole raw material can be screened through the first mesh sieve, so that the particle size of the obtained metronidazole is not greater than 300 microns. On the one hand, when the particle size of the raw material powder is too fine, the material will aggregate and affect the disintegration of the product, thereby affecting the dissolution; on the other hand, when the particle size of the raw material powder is too large, the effect of forming ...
Embodiment 1
[0089] Metronidazole is not pulverized and sieved, the D90 is about 300 microns, and the D90 of calcium hydrogen phosphate is about 200 microns.
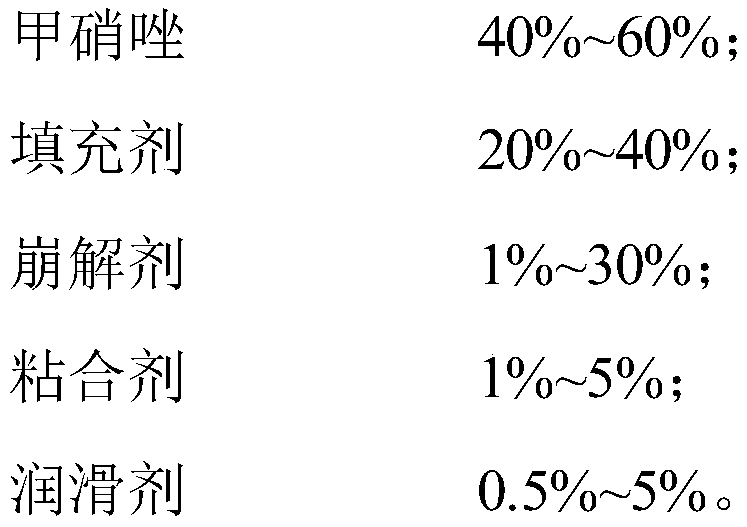
[0090] Taking the total mass of the metronidazole tablet as 100%, the composition of the mass percentage that the metronidazole tablet includes is:
[0091]
[0092] Metronidazole tablet preparation method is as follows:
[0093] Step S601: Take the metronidazole raw material for later use.
[0094] Step S602: Weigh the metronidazole, filler and disintegrant according to the preset mass percentage, mix them uniformly in a wet granulator, add the binder for less than 5 minutes to granulate, and pass through a 20-mesh sieve to obtain wet granules , wherein the particle size of the filler is 200 microns.
[0095] Step S603: drying the wet granules, the drying temperature is not higher than 60° C., and the moisture content of the dried granules is 2.5%.
[0096] Step S604: After the wet granules are dried, the granules are sized, ...
Embodiment 2
[0101] Metronidazole is not pulverized and sieved, the D90 is about 250 microns, and the D90 of calcium hydrogen phosphate is about 120 microns.
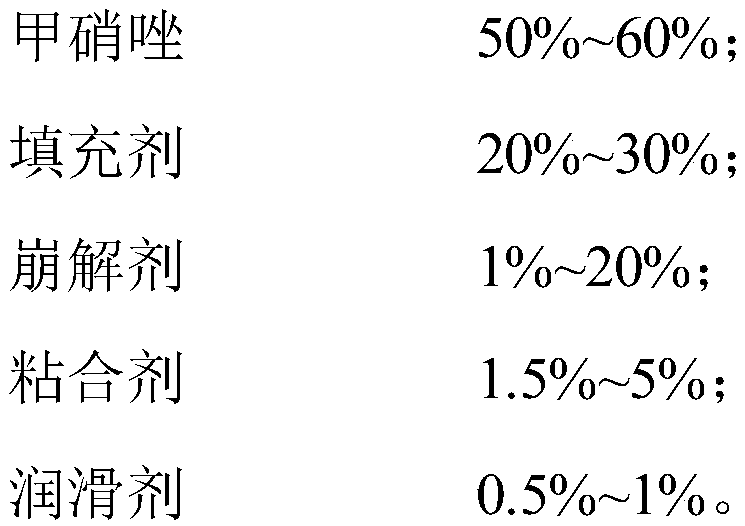
[0102] Taking the total mass of the metronidazole tablet as 100%, the composition of the mass percentage that the metronidazole tablet includes is:
[0103]
[0104] Metronidazole tablet preparation method is as follows:
[0105] Step S701: Pulverize the metronidazole raw material, pass through a 60-mesh sieve, and set aside.
[0106] Step S702: Weigh the metronidazole, filler and disintegrant according to the preset mass percentage, mix them uniformly in a wet granulator, add the binder for less than 5 minutes to granulate, and pass through a 20-mesh sieve to obtain wet granules , wherein the particle size of the filler is 200 microns.
[0107] Step S703: drying the wet granules, the drying temperature is not higher than 60° C., and the moisture content of the dried granules is 2.6%.
[0108] Step S704: After the wet granules...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com